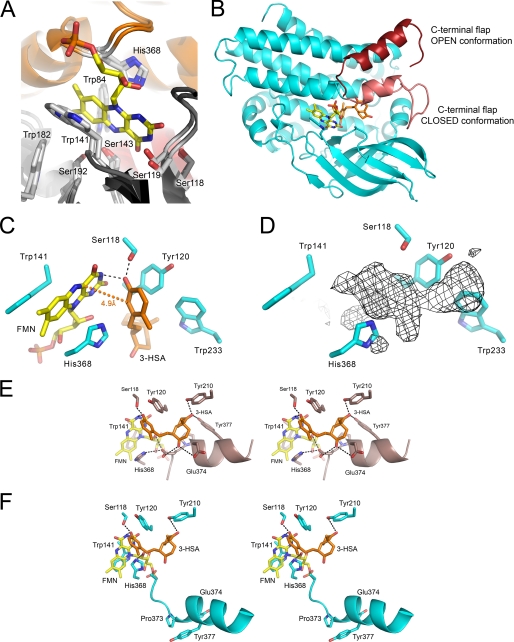
FIGURE 6.
The substrate-binding site of HsaA from M. tuberculosis. A, the HsaAO structure, in which FMN was placed in the active site based on a superposition with the pHPAH·flavin·pHPA ternary complex (PDB code: 2JBT). B, the modeled HsaA·flavin·3-HSA ternary complex showing the relative position and orientation of the 3-HSA (orange) and FMNH− (yellow) in the active site. The two conformations of the C-terminal flap (Val367–Val394) are shown in different shades of red. C, the relative orientation and geometry between the phenolic ring of 3-HSA and the isoalloxazine ring of the flavin in the modeled HsaAC·flavin·3-HSA complex. The distance between the phenolic C4 and flavin C4a atoms, 4.9 Å, is indicated by an orange dotted line. Predicted hydrogen bonds are indicated by black dashed lines. D, the same orientation of the active site as in C with the unassigned electron density in HsaAC. The wire mesh represents an Fo − Fc map contoured at 3σ. Stereo images of the bound substrates in modeled (E) HsaAC and (F) HsaAO complex highlight the interactions of the C9 and C17 ketones of 3-HSA. The depicted helix is part of the C-terminal flap.