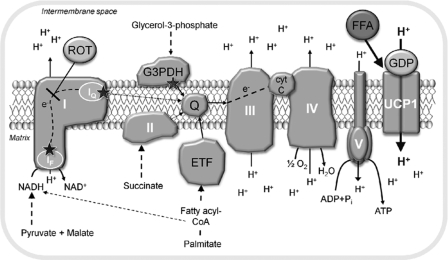
FIGURE 1.
Sites of substrate entry and superoxide production in the respiratory chain of BAT mitochondria. NADH-linked substrates donate their electrons to complex I. Further substrate entry sites, which are investigated in this study, are the mitochondrial glycerol-3-phosphate dehydrogenase (G3PDH), the succinate dehydrogenase (complex II), and the electron transfer flavoprotein (ETF), which is fueled by β-oxidation respectively. β-Oxidation also generates NADH, which enters complex I in parallel. The electrons from the entry complexes are transferred to ubiquinone (Q), forming QH2. In the normal electron forward transfer, the electrons are donated to complex III and then complex IV before finally reducing oxygen to water. Electrons may leak from the IF site and the IQ site of complex I. These sites can be distinguished by using the complex I inhibitor rotenone (ROT). A substantial amount of superoxide production can also derive from the glycerol-3-phosphate dehydrogenase and the electron transfer flavoprotein. The sites of superoxide production are indicated with stars in the figure. Proton motive force, generated by complexes I, III, and IV, is dissipated by either the ATP synthase (complex V) or UCP1. UCP1 is activated by free fatty acids (FFA) and inhibited by purine nucleoside di- and triphosphates (here, GDP).