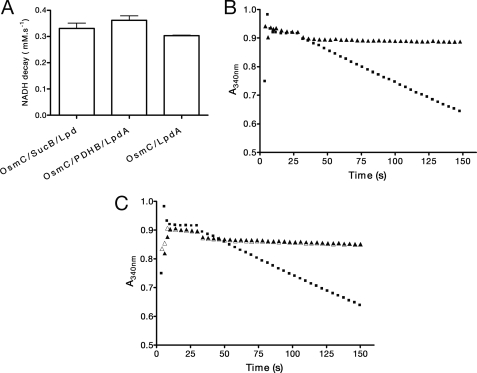
FIGURE 4.
Lipoyl-dependent peroxidase activity of OsmC from E. coli. A, OsmC peroxidase activity in the presence of lipoylated recombinant proteins from X. fastidiosa (LpdA (2 μm), PDHB (1 μm), and SucB (2 μm)). Reaction mixtures contained NADH (0.2 mm), Lpd (2 μm), and OsmC (0.5 μm). B, peroxidase activity of OsmC (10 μm, ▴) and PrxQ (5 μm, ■) from X. fastidiosa in the presence of the thioredoxin system from E. coli (thioredoxin (1 μm) and thioredoxin reductase (0.5 μm)). C, peroxidase activity of Ohr (▵, 10 μm), OsmC (▴, 10 μm), and human glutathione peroxidase (■, 1 μm glutathione peroxidase) in the presence of a heterologous glutathione system (6 μg/μl glutathione reductase, 1 mm GSH). Reaction mixtures were added as described under “Experimental Procedures.” The results presented in a are the average of three independent experiments. Data are means ± S.D. (error bars).