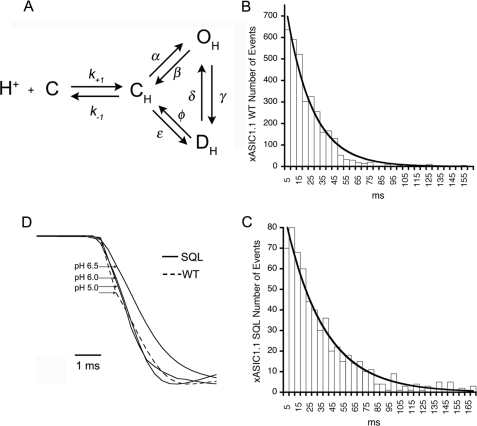
FIGURE 4.
The β1-β2 linker stabilizes a closed conformation of ASIC1. A, scheme of the simplest mechanism of ASIC1, where C is the closed nonprotonated state, CH represents the ensemble of different conformations the channel undergoes as it binds protons prior to opening. At least three protons are needed to open the channel, and nine protons are needed for steady-state desensitization according to the Hill coefficients of obtained in Fig. 1C. OH is the open protonated state, and DH is the desensitized state. The rate constants are denoted by the symbols on the arrows, and k−1/k+1 is the equilibrium constant for the initial binding step of protons. B and C, histograms of open times of wild type and mutant SQL xASIC1.1. The lines are fits of the data to single exponentials. D, rising phase of averaged current responses from patches with wild type (WT) or SQL xASIC channels. Currents are scaled to the same peak current value. The region of the onset curve >20% of maximum is well described by a single exponential function. This part of the curve is determined primarily by the channel opening rate rather than agonist binding so that the time constant of the exponential function approximates the reciprocal of the opening rate. Onset times were as follows: 2 ms at pH 6.5, 1.5 ms at pH 6.0, and 1.4 ms at pH 5.0.