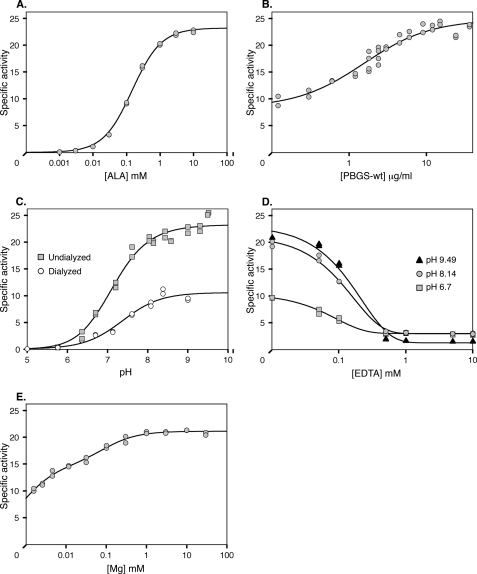
FIGURE 2.
Kinetic properties of wild type TgPBGS. A, specific activity as a function of ALA concentration. Fitting to the Michaelis-Menten equation yields a Km value of 0.149 mm and Vmax of 23.2 μmol porphobilinogen/mg of protein/h. B, TgPBGS specific activity is dependent on enzyme concentration, reaching maximal activity at ∼10 μg/ml (∼0.25 μm). The data are fitted to a simple hyperbolic equation with a y (offset). C, specific activity of TgPBGS as a function of pH and Mg2+. Undialyzed protein (squares) contains ∼2 μm Mg2+, and dialyzed protein (circles) contains <0.01 μm Mg2+; ALA contributed an additional ∼0.18 μm Mg2+ to the assay. D, effect of EDTA on TgPBGS enzyme activity (using undialyzed TgPBGS without added Mg2+). E, MgCl2 titration of TgPBGS specific activity. Two Kd values of ∼0.7 and 84 μm were determined, based on fitting a double hyperbolic equation. Unless otherwise specified, standard assay conditions included 10 μg/ml TgPBGS (except in B), 10 mm ALA (except in A), pH 8.14 (except in C and D), 1 mm MgCl2 (except in C–E), and 10 mm β-ME; assays were initiated by the addition of ALA and incubated for 20 min at 37 °C.