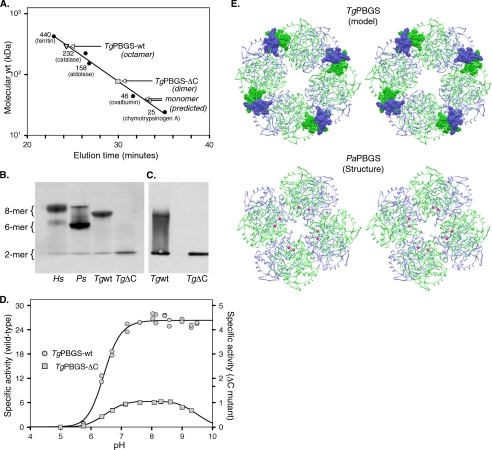
FIGURE 3.
Quaternary structure analysis of TgPBGS-wt and TgPBGS-ΔC. A, determination of molecular mass by gel filtration chromatography on Superdex 200 (0.1 m Tris, pH 8.5, 10 mm MgCl2, 10 mm β-ME, 0.2 m NaCl). Enzyme elution was assessed at A280, and the column was calibrated using size markers as shown (Amersham Biosciences). For comparison, the expected elution times for monomeric TgPBGS-wt and TgPBGS-ΔC are also indicated. B, native PAGE analysis of HsPBGS (octamer > hexamer ≫ dimer), PsPBGS (hexamer > octamer ≫ dimer), TgPBGS-wt (octamer ≫ dimer), and TgPBGS-ΔC (dimer only). C, Western blotting (of native gels) confirms that the TgPBGS-ΔC mutant fails to form octamers. Comparison with B suggests that this antibody displays higher affinity for the dimer than the octamer form of TgPBGS. D, activity profile demonstrates ∼25-fold lower specific activity for TgPBGS-ΔC relative to TgPBGS-wt (0.1 m Bistris propane, 1 mm MgCl2, 10 mm β-ME, 10 mm ALA; pH range as indicated). E, homology model of TgPBGS octamer (top) and crystal structure of PaPBGS octamer (Protein Data Bank entry 1GZG; bottom), displayed as stereo images. Green, subunits A, C, E, and G; blue, subunits B, D, F, and H. Magenta, allosteric magnesium ions stabilizing the octamer form of PaPBGS. TgPBGS octamers are stable even in the absence of magnesium, probably because of dimer-dimer interactions mediated by the C-terminal extension unique to apicomplexan parasites (amino acids 646–658; shown in CPK).