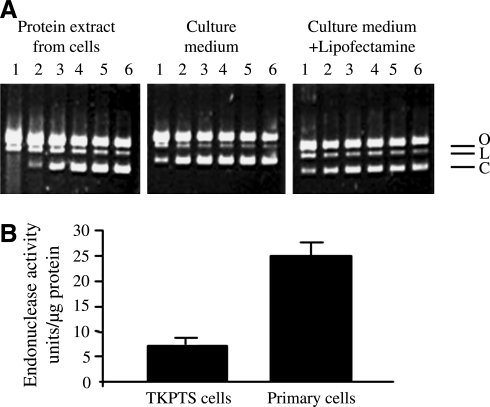
FIG. 1.
Endonuclease activity in tubular epithelial cell extract and culture medium. The activity was measured using pBR322 PIA as described in the Materials and Methods section. (A) Endonuclease activity is present both in the cellular protein extracts and in the culture medium (left and middle panels). Pretreatment with Lipofectamine does not protect plasmid DNA against in vitro digestion by endonucleases (right panel). Dilutions (1–6) of cell extract or medium 1:1, 1:5, 1:25, 1:125, 1:625, and 1:3125, respectively. O, open circular DNA (with one or more single-strand DNA breaks but no double-strand breaks); L, linear DNA (with one double-strand DNA break); C, covalently closed circular DNA (without DNA breaks), which is the primary substrate for endonucleases. Endonuclease activity is seen only in the first two dilutions in cell extract, and in nondiluted culture medium. (B) Endonuclease activity in immortalized TKPTS cells and PTE cells. Primary cells have a higher total endonuclease activity (25 ± 2 units/μg protein in primary cells vs. 7 ± 3 units/μg protein in TKPTS cells, n = 3–6, p < 0.001) as measured using the pBR322 PIA in the presence of Ca2+ and Mg2+ ions (2 mM CaCl2 and 5 mM MgCl2), which are the cofactors for most of the cellular endonucleases. PIA, plasmid incision assay; TKPTS, mouse kidney proximal tubule epithelial; PTE, primary tubular epithelial.