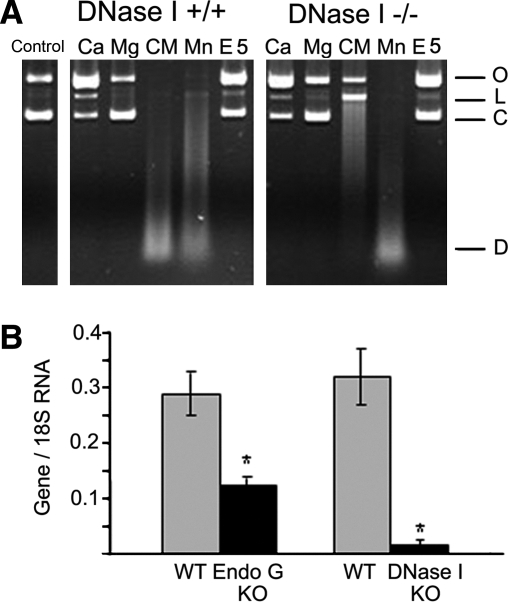
FIG. 2.
Activity and expression of endonucleases in PTE cells. (A) In the total protein extracts isolated from DNase I WT mice, the strongest endonuclease activity could be obtained when Ca2+ and Mg2+ ions were added together, resulting in digested DNA. This is characteristic to DNase I, which therefore provides most of the endonuclease activity in the normal kidney. In the kidney tissue extracts obtained from DNase I KO mice, Mn-dependent endonuclease was the most prominent, suggesting that EndoG is the second major endonuclease in the absence of DNase I (Widlak et al., 2001). Vertical row: O, open circular DNA; L, linear DNA; C, covalently closed circular DNA; D, digested DNA. Horizontal row: control nondigested pBR322 DNA; Ca2+, 2 mM CaCl2, pH 7.5; Mg2+, 2 mM MgCl2, pH 7.5; CM [Ca2++Mg2+], 2 mM CaCl2 + 2 mM MgCl2; Mn2+, 2 mM MnCl2, pH 7.5; E5, 2 mM EDTA, no cations, pH 5 (to measure DNase II activity). (B) Expression of endonucleases in WT, EndoG KO, and DNase I KO cells measured using real-time reverse transcriptase polymerase chain reaction. DNase I expression is wiped out almost completely, while EndoG KO is partially inhibited because these cells were isolated from heterozygous animals (n = 4, *p < 0.001). DNase I, deoxyribonuclease I; WT, wild-type; KO, knockout; EndoG, endonuclease G.