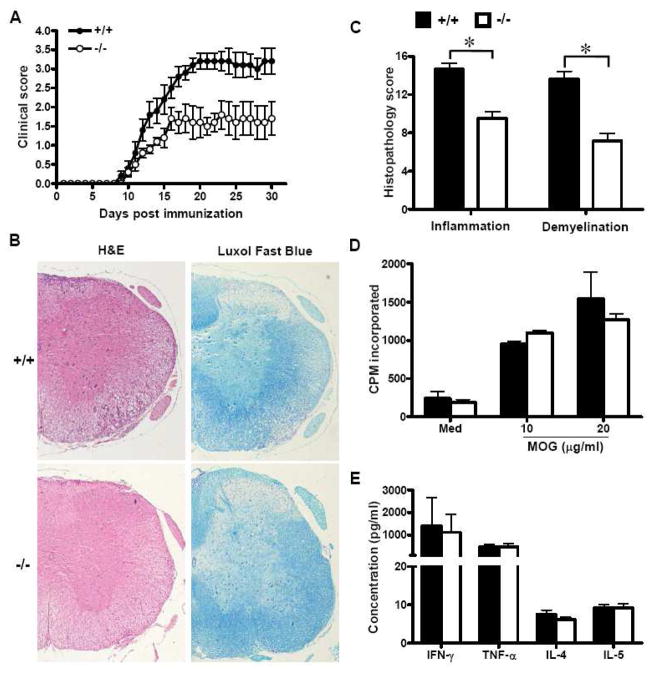
FIGURE 2.
SRC-3 deficiency attenuates MOG35-55-induced EAE without impairment of peripheral T cell response. (A) Clinical course and severity of EAE were assessed after immunization with MOG35–55 in SRC-3+/+ (n=10, closed circle) and SRC-3−/− mice (n=10, open circle). Mice were monitored and scored daily as described in Materials and Methods. Data are representative of three independent experiments. (B) Histopathology of spinal cord tissue sections from SRC-3+/+ and SRC-3−/− EAE mice by H&E and Luxol Fast Blue staining (×40). (C) Semiquantitative analysis of inflammation and demyelination in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice. *P < 0.05. (D) Splenocytes were isolated from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization and examined ex vivo for proliferation in the absence (Med) or indicated concentration of the MOG35-55 peptide. Data are presented as mean ± SD in triplicates. (E) Splenocytes from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization were challenged with the MOG35-55 peptide (20 μg/ml), and culture supernatants were collected at 48 h for cytokine measurement by ELISA. Data are presented as mean mean ± SD of triplicate samples.