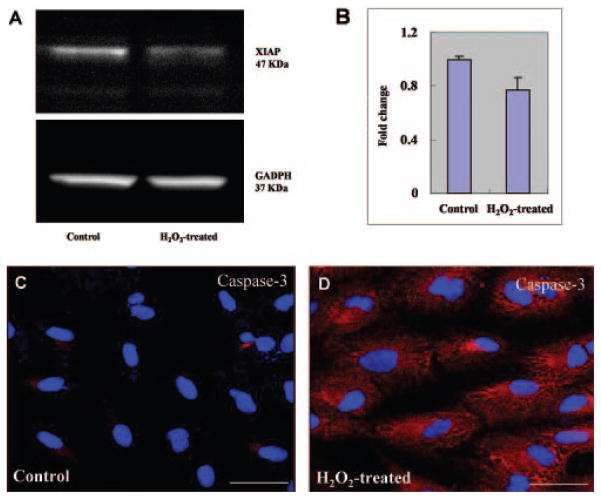
Figure 3.
XIAP degradation and caspase-3 activation after H2O2-induced oxidative stress. (A) Total protein samples were prepared as described in Figure 2 and were detected by immunoblot with rabbit anti-XIAP antibody or GAPDH (loading control). XIAP was decreased in H2O2-induced cells compared with that of untreated cells, indicating that release of mature HtrA2 into the cytosol leads to cleavage and inactivation of XIAP, an inhibitor of apoptosis, thus triggering the cascade of apoptosis. (B) Quantification of XIAP protein levels are represented graphically. (C, D) Cells were fixed after incubation with 1 mM H2O2 for 2 hours and then stained with rabbit anti-human caspase-3 (red). The nuclei were stained with DAPI (blue). The intensities of immunostaining for the activated subunits of caspase-3 were higher in H2O2-treated cells (D) than in untreated control cells (C). Scale bar, 50 μm.