Abstract
Perineal muscles essential for copulatory functioning are innvervated by Onuf’s nucleus in humans and the spinal nucleus of the bulbocavernosus (SNB) and dorsolateral nucleus (DLN) in rats. These structures sexually differentiate as a result of developmental androgen exposure in most species examined. The homologous structure in the Asian musk shrew (Suncus murinus) is a single cluster in the lateral DLN/Onuf’s position in the ventral horn of the spinal cord; these motoneurons innervate both the bulbocavernosus and ischiocavernosus muscles of the musk shrew. We found the expected sex difference in motoneuron number in the shrew DLN, but not in two neighboring motoneuron clusters, the retrodorsolateral nucleus (RDLN) and ventrolateral nucleus (VLN). Male musk shrews also have significantly larger soma areas in the VLN and DLN than females, and male DLN motoneurons have significantly larger nuclei than female. The sex difference in DLN motoneuron number was evident both in raw counts and after accounting for split nuclei error.
1. Introduction
Motoneurons that innervate sexual differentiated perineal muscles, the levator ani (LA), the bulbocavernosus (BC) and the ischiocavernosus (IC) are sexually dimorphic in a variety of mammals, including rats, mice, dogs, hyenas and humans (Sengelaub and Forger, 2008, review). In rats, the BC/LA and IC motoneurons are segregated into separate clusters, with the BC and LA innvervated by the dorsomedially positioned spinal nucleus of the bulbocavernosus (SNB) and the IC by more lateral nucleus at the same levels of the spinal cord, the dorsolateral nucleus (DLN, Jordan et al., 1982). In most non-rodent mammals studied (e.g. dog, hyena, human) all three muscles are innervated by one cluster called Onuf’s nucleus (Forger and Breedlove, 1986; Forger et al., 1996). Onuf’s nucleus is of clinical interest to in humans because of its apparent resistance to some neurogenerative diseases and vulnerability in others (Pullen et al., 1997).
In rats, the sex difference in SNB and DLN motoneuron number rises during development, when androgen receptor activation at the target muscles promotes their survival in males; the muscles in turn secrete trophic factors that enable the motoneuron’s survival (Sengelaub and Forger, 2008; Jordan et al, 1982; Tobin and Payne, 1991). The mechanism for sexual differentiation of this system in other mammals has not been as extensively studied, but is hypothesized to occur by similar means.
Asian musk shrews (Suncus murinus) are of the order Insectivora and thought to resemble the earliest placental mammals. Aspects of sexual differentiation in the musk shrew, specifically the role of reduced testosterone metabolites in behavioral masculinization, more closely resemble primates than laboratory rodents (Ewton et al., 2010). Genetic evidence also suggests that the Asian musk shrew is more closely related to humans than are other common laboratory animals. (Hoyle et al., 2003). Since the Onuf’s nucleus homologue had never previously been studied in an insectivore model, we localized motoneurons innervating the BC, IC, external anal sphincter (EAS), another target muscle of the SNB in rats and Onuf’s nucleus in humans and primate, (Mckenna and Nadelhaft, 1986) and the flexor digitorum brevis (FDB), innervated by the sexually monomorphic retrodorsolateral nucleus (RDLN, Leslie et al., 1991) in rats. We evaluated the motor pool containing perineal motoneurons, as well as two adjacent motor pools for sex differences in motoneuron number, soma area and nuclear area.
2. Results
2.1 Spinal cord organization
Examination of Nissl-stained tissue found that four distinct motoneuron pools were visible at the 5th and 6th lumbar levels of the musk shrew spinal cord (Fig 1). Motoneurons labeled by BC and IC injections are shown in Fig. 2. The majority of cells labeled by horseradish peroxidase (HRP) injections into the BC and IC were found in a motoneuron cluster (labeled DLN, Fig. 1) in the lateral part of the ventral horn, in a position reminiscent of the DLN of rats and Onuf’s nucleus in carnivores (Forger and Breedlove, 1986; Forger et al. 1996). Injections of the EAS also filled motoneurons in this group (Fig. 3a). A small number of labeled BC and IC motoneurons (< 10 per animal, typically in the caudalmost sections of the lumbar spinal cord) were also seen at the small, medially located cluster at the bottom of the ventral horn (medial nucleus, MN, Fig. 1, see also Fig 2A and 2C). Two other motoneuron clusters, one dorsal and one ventral to the shrew DLN are also seen at this level (Fig. 1). The dorsalmost cluster contained motoneurons labeled by FDB in both sexes (Fig 3B); we refer to this cluster as the RDLN (Fig. 1) in accordance with the terminology used in rats (Leslie et al., 1991). The ventrolateral nucleus (VLN) did not show any labeled motoneurons from any of the four muscles we injected.
Figure 1. Four motoneuron pools in the musk shrew lumbar spinal cord.
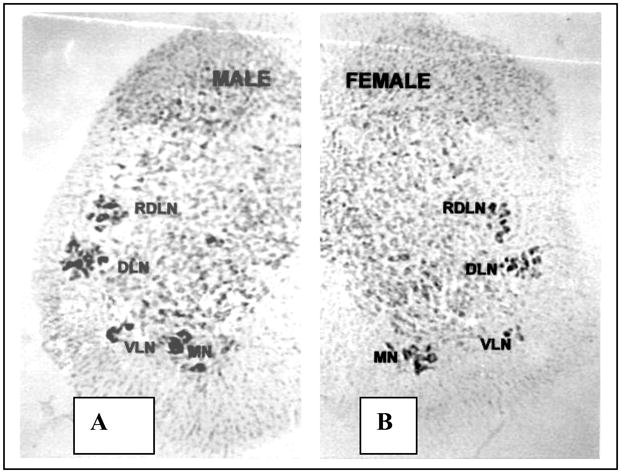
The layout of motor pools in the lumbar spinal cord of the male (left) and female (right) musk shrew. The sexually dimorphic bulbocavernosus (BC) and ischiocavernosus (IC) muscles are innervated by the dorsolateral nucleus (DLN) in males, which also innervates the external anal sphincter in both sexes. This cluster seems the homologus to Onuf’s nucleus in other species. A few BC and IC motoneurons were also found in the medial nucleus (MN). The retrodorsolateral nucleus (RDLN) innervates the flexor digitorum brevis in both sexes, while the target muscles of the ventrolateral nucleus VLN are not known.
Figure 2. Motoneurons labeled by horseradish peroxidase (HRP) injections of the ischiocavernosus (IC, left) and bulbocavernosus (BC, right).
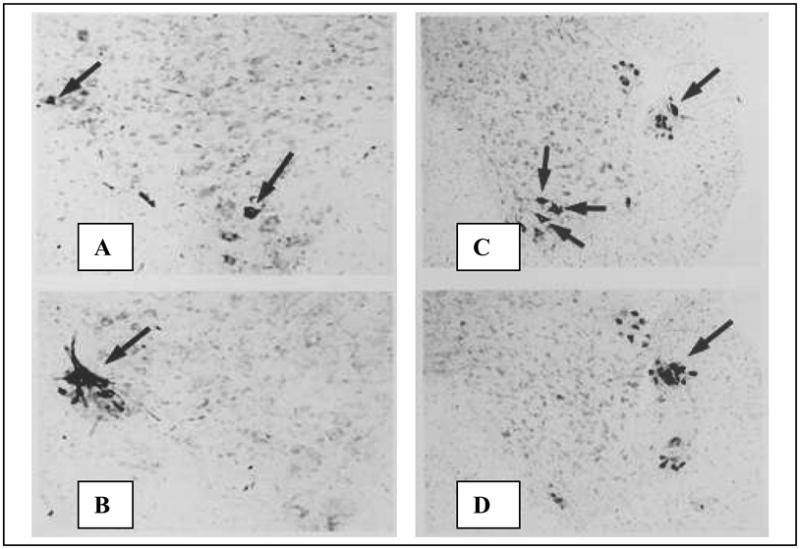
Fig. 2A: High magnification view of the left ventral horn of an L6 segment of a male shrew after HRP injection of the IC. Arrows indicate HRP-filled motoneurons in the dorsolateral nucleus (DLN) and the medial nucleus (MN). No ventrolateral nucleus (VLN) motoneurons are visible in this section. Fig. 2B. More rostrally, the same injection labels DLN motoneurons only. Fig. 2C: Lower magnification photo of the right ventral horn in the L6 spinal cord of a male shrew injected in the BC muscle. HRP-filled neurons are seen in the DLN and MN. Fig. 2D: More rostrally in the same animal, cells are labeled only in the DLN.
Figure 3. Motoneurons labeled by horseradish peroxidase (HRP) injections of the external anal sphincter (EAS, top) and flexor digitorum brevis (FDB, bottom).
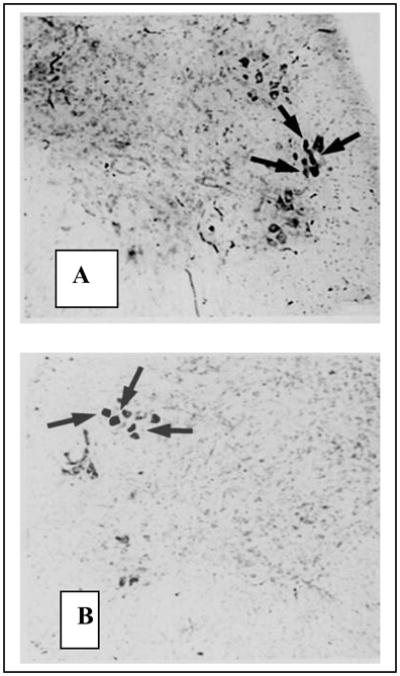
Fig. 3A: HRP injection of the EAS, a muscle present in both sexes, labeled motoneurons in the shrew DLN. Unlabeled neurons are visible in the retrodorsal lateral nucleus (RDLN) above and the vetrolateral nucleus (VLN) below. Fig. 3B. As is the case for rats and mice, HRP-injections of the FDB of the musk shrew label motoneurons in the RDLN but not the DLN.
Because the MN contained relatively few labeled motoneurons compared to the other three clusters, we evaluated sex differences in only the DLN, RDLN and VLN.
2.2 Motoneuron number
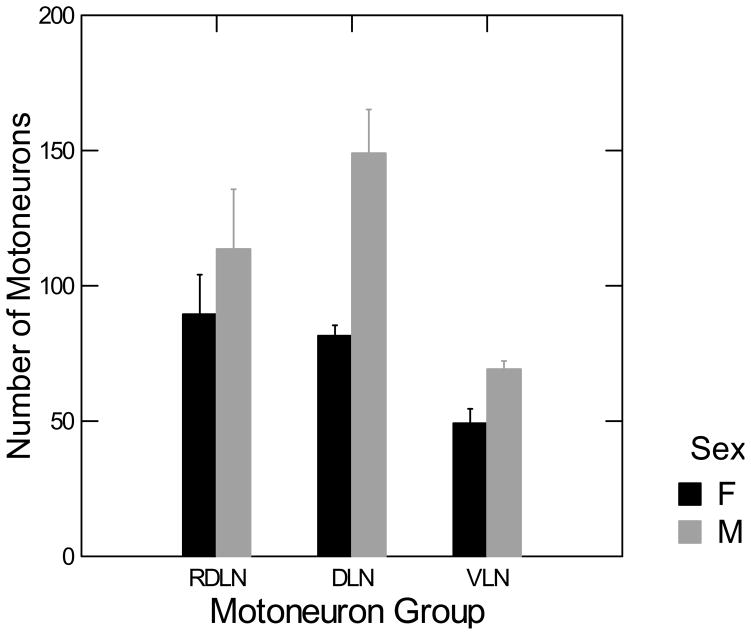
Count-corrected motoneuron numbers in the RDLN, DLN, and VLN for males and females are shown in Figure 4. The interaction between sex and motoneuron groups was significant, [F(2,18) = 3.967, p < 0.05]. Post –hoc Fisher’s protected t-tests [t(18) = 5.04, p < 0.05] indicate that males have significantly more motoneurons than females in the DLN, but not the RDLN or VLN.
Figure 4. Sex difference in motoneuron count in the DLN.
Two-way ANOVA (sex by nucleus) followed by planned comparisons showed that only the DLN was sexually dimorphic in motoneuron number. Error bars indicate SEM.
2.2 Soma area
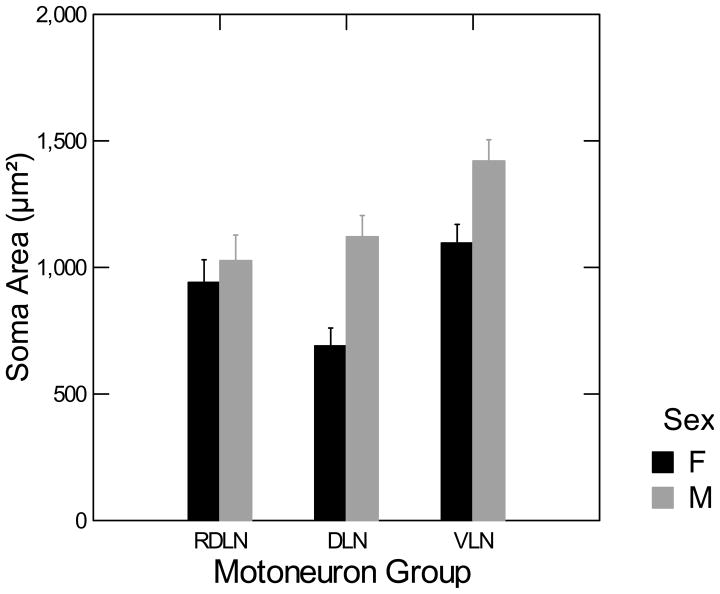
The average soma area of the RDLN, DLN, and VLN motoneurons for males and females is shown in Figure 5. The interaction between gender and motoneuron groups is significant, [F(2,18) = 4.970, p <0.05]. Fisher’s protected t-tests indicate that males have significantly greater soma areas in the DLN [t(18) = 5.44, p < 0.05] and VLN [t(18) = 4.08, p < 0.05] than females. There was no sex difference in the motoneuron soma area in the RDLN.
Figure 5. Sex difference in motoneuron size in the DLN and VLN.
Two-way ANOVA (sex by nucleus) followed by planned comparisons showed that the largest sex difference in motoneuron size was in the DLN. The VLN also had bigger motoneurons in males than in females; RDLN had no sex difference. Error bars indicate SEM.
2.3 Nuclear area
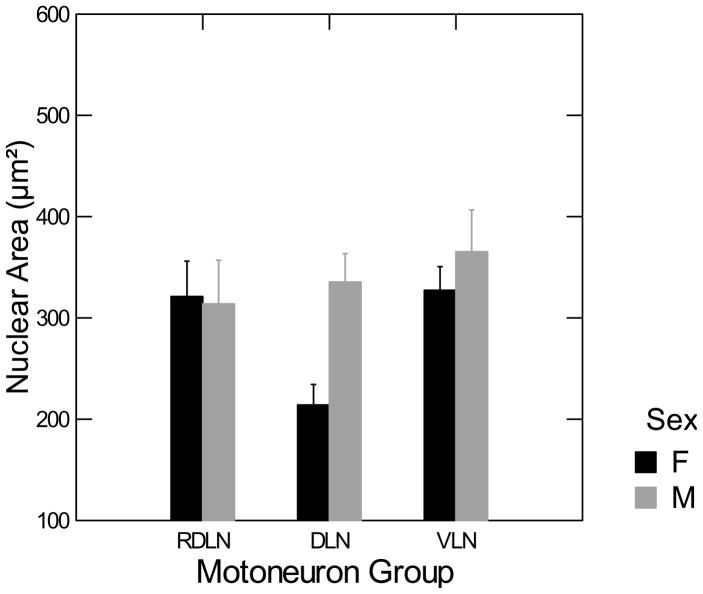
The nuclear area of the RDLN, DLN, and VLN motoneurons for males and females is shown in Figure 6. The interaction between gender and motoneuron groups was significant [F(2,18) = 4.620, p <0.05]. Post-hoc Fisher’s protected t-tests indicate that males have significantly larger nuclei in the DLN [t(18) = 4.00, p < 0.05] than females, but there is no sex difference in the RDLN and VLN.
Figure 6. Sex difference in motoneuron nuclear area in the DLN.
Two-way ANOVA (sex by nucleus) followed by planned comparisons showed that motoneuron nuclei were larger in males than in females only in the DLN. Error bars indicate SEM.
Motoneuron number, soma area and nuclear areas are summarized in Table 1.
Table 1.
Mean (± SEM) corrected motoneuron counts, soma area and nuclear areas of three motor pools in the lumbar spinal cord of male and female musk shrew.
| VLN | DLN | RDLN | |
|---|---|---|---|
| Motoneuron count | M: 48.884 ± 3.245 F: 37.550 ± 2.962 |
M: 106.671 ± 7.095 F: 62.244 ± 6.477* |
M: 82.155 ± 12.116 F: 64.505 ± 11.060 |
| Soma area (square μm) | M: 1420.380 ± 72.805 F: 1096.459 ± 66.462* |
M: 1120.949 ± 71.298 F: 689.634 ± 65.086* |
M: 1026.667 ± 88.356 F: 940.768 ± 80.658 |
| Nuclear area (square μm) | M: 365.343 ± 29.560 F: 327.310 ± 26.984 |
M: 335.420 ± 22.128 F: 214.146 ± 20.200* |
M: 313.871 ± 35.940 F: 321.045 ± 32.809 |
Significantly different from males, p < 0.05, Fisher’s protected t-test.
VLN= ventrolateral nucleus; DLN=dorsolateral nucleus, RDLN = retrodorsolateral nucleus. The DLN innervates the sexually dimorphic perineal muscles and is the only motor pool to show a sex difference in motoneuron number.
3. Discussion
Retrograde labeling showed that the shrew DLN innverates two known target muscles of the rat SNB (the BC and EAS muscles) and one of the rat DLN (the IC muscle). While most rodents segregate BC and IC motoneurons into separate nuclei (Freeman and Breedlove, 1995; Jordan et al, 1982; Tobin and Payne, 1991) musk shrews resemble carnivores in the organization of most BC and IC motoneurons into a single laterally placed cluster, usually called Onuf’s nucleus (Forger and Breedlove, 1986; Forger et al., 1996). Interestingly, Onuf’s nucleus in humans is found in a more ventromedial position, similar to the shrew MN, which also contained a few BC and IC-innervating motoneurons (Fig. 2A and 2C). All three of these muscles (BC, IC, EAS), along with the LA and the external urethral sphincter (EUS) are innervated by the pudendal nerve; the soma of motoneurons innervating these muscles are found in Onuf’s nucleus in carnivores and primates. It is likely that the shrew DLN is homologous to this system; however, we cannot rule out the possibility that LA and EUS neurons are found elsewhere in the shrew spinal cord.
Our results confirm our hypothesis that male shrews have significantly more DLN, but not RDLN or VLN motoneurons than females. Sexual dimorphism in the BC- and IC-innervating motoneurons has been found in multiple species in every mammalian order examined to date; therefore, it is not surprising that an insectivore would also show this dimorphism. Our results are further indication that sexual dimorphism and hormone sensitivity in this neuromuscular system is likely a highly conserved evolutionary trait, with only rare exceptions in unusual mammals (e.g. naked mole-rats, Peroulakis et al., 2002).
Musk shrews represent a seldom-studied order thought to resemble the earliest ancestral placental mammals that underwent adaptive radiation after the extinction of the dinosaurs (Butler, 1988). The phylogeny of the insectivores remains controversial (Symonds, 2003), but genetic evidence based on mitochondrial DNA suggests that primates and insectivores are phylogenetically closer than are primates and rodents (Stanhope et al., 1998). Analysis of pancreatic polypeptide cDNA suggests that musk shrews specifically are more closely related to humans than are common laboratory animals such as rats, rabbits, and guinea pigs (Hoyle et al., 2003). While the role of early androgenic hormone action in differentiating the SNB and DLN has been most clearly elucidated in the rat (Sengelaub and Forger, 2008; Tobin and Payne, 1991), comparative studies suggest similar mechanisms in carnivores. Early testosterone treatment of female dogs eliminates the sex difference in Onuf’s nucleus (Forger and Breedlove, 1986); while androgen receptor blockers given prenatally to male hyena pups reduce cell number in Onuf’s nucleus (Forger et al., 1996). Whether sexual dimorphism in the human Onuf’s nucleus arises through a similar pattern of hormone-regulated cell death is unknown, though that mechanism is possible given that fetal motoneuron apoptosis coincides with the prenatal testosterone surge that contributes to phenotypic masculinization in humans (Forger and Breedlove, 1987). Future studies will examine the effects of early androgen treatment of female musk shrews; it is predicted that androgen will promote retention of both the perineal muscles and motoneurons.
Sex differences in motoneuron size, though also widespread in mammals and frequently influenced by androgens, are believed to arise through independent mechanisms and are likely a direct effect on the motoneuron rather than an indirect effect through muscle-derived trophic factors (Freeman and Breedlove, 1995; Watson et al, 2001; Zuloaga et al., 2007). This effect is not limited to motoneuron clusters innervating primarily sexually differentiated and especially androgen-sensitive muscles or to ones in which males have more motoneurons than females. The homologous system in the guinea pig shows sex differences in motoneuron size but not number (Freeman and Breedlove, 1995). The RDLN motoneurons of rats (Leslie et al., 1991) but not mice (Zuloaga et al. 2007) increase in size with adult androgen treatment. In the musk shrew, we saw the largest sex difference in motoneuron size in the DLN, a smaller sex difference in the VLN (whose target muscles are unknown) and none in the RDLN. Adult sexual receptivity in the female musk shrew is maintained tonically by andrenal- and ovarian-derived testosterone rather than the cyclic release of estrogen and progesterone from the ovaries (Rissman and Bronson, 1987; Rissman and Crews, 1988); however, circulating testosterone levels are still about 5X higher in male than female shrews (Fortman et al, 1992; Rissman, 1987; Rissman and Crews, 1988). It is therefore possible that sex differences in adult testosterone levels contribute to the sex difference in motoneuron size in the musk shrew despite the unusual pattern activation of both female and male reproductive behaviors by testosterone.
Nuclear area was sexually dimorphic only in the VLN. Since larger motoneuron nuclei can lead to split nuclei error and overestimates of motoneuron number (Konigsmark, 1970), it is important to compensate for that with a correction formula. In guinea pigs both soma area and nuclei are larger in males than in females (Freeman and Breedlove, 1995), but the sex difference in the musk shrew DLN persisted after count correction. Formulaic count correction of motoneuron number is an imperfect estimate; however, the persistence of sexual dimorphism in the DLN, but not adjacent clusters in the same spinal section, is evidence for a genuine sex difference in that motor pool when evaluated using equivalent methods of count correction.
Given its hypothesized close phylogenetic relationship to humans (Hoyle et al., 2003) and its convenience as a small mammal, the musk shrew could prove a useful laboratory animal model to test the applicability of insights gained from the rat and mouse SNB system to the human Onuf’s nucleus. Correlations have been found between certain human pathologies in the Onuf’s nucleus and specific central nervous system disorders. Onuf’s nucleus seems less vulnerable to amyotrophic lateral sclerosis (Kihira et al., 1991; Mannen et al., 1982) but undergoes a significant loss of motoneurons in patients with progressive supranuclear palsy (Winge et al, 2010). Onuf’s nucleus also shows targeted damage in Shy-Drägers syndrome (Mannen et al., 1982). An understanding of this evolutionarily conserved motoneuron system is integral to the understanding of not only of sexual differentiation but possibly degenerative disorders of the central nervous system, particularly movement disorders.
4. Experimental Method
4.1 Localization of motoneurons
Retired breeders were euthanized with sodium pentobarbital overdose. Gross dissection showed that the BC and IC muscles were present male but not female musk shrews; EAS and FDB muscles were present in both sexes. To localize the motoneurons innervating these muscles, retired breeders (1–3 for each muscle) were anesthetized with sodium pentobarbital and the muscle of interest injected bilaterally with 30% HRP (Sigma Type IV in d H2O, 5 μl per side). Two days later, the animals were overdosed with sodium pentobarbital then perfused intracardially with physiological saline followed by 1% paraformaldehyde-glutaraldehyde. Spinal cords were removed, post-fixed 1–3 hours then soaked overnight in 20% buffered sucrose. Cords were frozen-sectioned on the coronal plane at 50 μm. Sections were reacted with tetramethyl benzidine to visualize HRP (Mesulam, 1978) then counterstained with neutral red. Surgical, anesthesia and euthanization procedures were Institutional Care and Use Committee-approved and consistent with NIH guidelines.
4.2 Quantification of sex differences
We examined the spinal cords of 6 female and 5 male Asian musk shrews (Suncus murinus) 6–12 months in age from the Mary Baldwin College colony. Animals were raised in an environmentally controlled room with temperature 68–74 F, humidity 40–70%, light cycle 14L:10D and ad lib (10 parts Purina Cat Chow to 1 part Milk Specialties GroFur mink chows and tap water). Shrews were housed singly in plastic cages (27 × 21 × 13 cm) with pine shaving bedding. These conditions were approved by the Institutional Care and Use Committee and are consistent with NIH guidelines Animals were euthanized with halothane and immediately perfused intracardially using a 0.9% phosphate buffered saline followed by 10% buffered formalin. The lumbar spinal cords were removed and post-fixed in formalin for a minimum of one week.
Twenty-four hours prior to sectioning, the spinal cords were placed in a 20% sucrose solution. A freezing microtome was used to section the spinal cords into fifty μm sections. Sections were mounted on gelatin-subbed slides and labeled with pencil. The slides were dried overnight and stained with thionin.
4.3 Counting and measurements
The sections containing the three distinct nuclei were located using a standard laboratory light microscope at 100X magnification. All motoneurons in each cluster with a visible nucleus were counted bilaterally at 400X magnification in consecutive caudal-rostral sections beginning with the caudalmost section in which all three clusters were visible until the three clusters become indistinguishable from one another. Motor pools were visible in 5–10 sections of males and 4–9 sections of females. The soma and nucleus of ten motoneurons per cluster per animal were outlined and area measured using ImagePro software. Pixel areas converted to square microns by measuring a 10 μm segment of a stage micrometer ruler (Ward’s, W9910) with ImagePro under the same magnification. All counts and measurements were made by an observer blind to the sex of the animals. Raw motoneuron counts were corrected for split nuclei error by a formula used elsewhere (Breedlove and Arnold, 1981; Freeman and Breedlove, 1995) and adapted from Konigsmark (1970). A split cell error refers to the fact that the nucleus of a cell could potentially be split during sectioning, which would overestimate the count of the motoneurons. The formula applied was
where N′ is the estimated neuron count, n is the raw motoneuron count, d is the average nuclear diameter, t is the section thickness (50 μm) and k is the diameter of the smallest recorded neuronal nucleus (Konigsmark, 1970). The average nuclear diameter was calculated by d = 2√(area/π), using the average nuclear size of each cluster. The smallest nuclear diameter was calculated by k = 2√(area/π), using the smallest nucleus measured in the study.
The corrected motoneuron counts, soma size and nuclear size of each motoneuron cluster were analyzed for significance with the use of a two-way mixed ANOVA (sex by motoneuron pool) using Systat statistical software. Planned comparisons used Fisher’s protected t-test.
Acknowledgments
The authors thank Ms. Julia Rhodes for her help with manuscript preparation. Supported by NIH 2 R15 HD043808-02A1 and 3 R15 HD043808-02A1S1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Butler P. Phylogeny of the insectivores. In: Benton M, editor. The phylogeny and classification of the tetrapods, Vol 2: Mammals. Clarendon Press; Oxford: 1988. pp. 117–141. [Google Scholar]
- Ewton TA, Siboni RB, Jackson A, Freeman LM. Neonatal DHT but not E2 speeds induction of sexual receptivity in the musk shrew. Physiol Behav. 2010;100:216–220. doi: 10.1016/j.physbeh.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: Role of early androgen. Proc Natl Acad Sci (USA) 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Motoneuronal death during human fetal development. J Comp Neurol. 1987;264(1):118–122. doi: 10.1002/cne.902640109. [DOI] [PubMed] [Google Scholar]
- Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol. 1996;375(2):333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Fortman M, Dellovade T, Rissman EF. Adrenal contribution to the induction of sexual behavior in the female musk shrew. Horm Behav. 1992;26:76–86. doi: 10.1016/0018-506x(92)90033-r. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Breedlove SM. Motoneurons innervating guinea pig perineal muscles are sexually dimorphic in size but not number. Brain Res. 1995;690:1–7. doi: 10.1016/0006-8993(95)00442-s. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Hill J, Sanger GJ, Andrews PL. Analysis of pancreatic polypeptide cDNA from the house musk shrew, Suncus murinus, suggests a phylogenetically closer relationship with humans than for other small laboratory animal species. Regul Pept. 2003;114:137–144. doi: 10.1016/s0167-0115(03)00113-7. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249:309–314. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Kihira T, Yoshida S, Uebvashi Y, Yase Y, Yoshimasu F. Involvement of Onuf’s nucleus in ALS: demonstration of intraneuronal conglomerate inclusions and Bunina bodies. J Neurol Sci. 1991;104(2):119–128. doi: 10.1016/0022-510x(91)90300-v. [DOI] [PubMed] [Google Scholar]
- Konigsmark BW. Methods for the Counting of Neurons. In: Nauta WJH, Ebbesson SO, editors. Contemporary Research Methods in Neuroanatomy. New York: Springer-Verlag; 1970. pp. 315–340. [Google Scholar]
- Leslie ML, Forger NG, Breedlove SM. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991;561(2):269–273. doi: 10.1016/0006-8993(91)91603-x. [DOI] [PubMed] [Google Scholar]
- Mannen T, Iwata M, Toyokura Y, Nagashima K. The Onuf’s nucleus and the external anal sphincter muscles in amoytrophic lateral sclerosis and Shy-Drager syndrome. Acta Neuropathol. 1982;58(4):255–260. doi: 10.1007/BF00688606. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248(4):532–548. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benezide for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural efferents and afferents. J Histochem Cytochem. 1978;26:498–504. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Peroulakis ME, Goldman B, Forger NG. Perineal muscles and motoneurons are sexually monomorphic in the naked mole-rat (Hetercephalas glaber) J Neurobiol. 2002;51(1):33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- Pullen AH, Tucker D, Martin JE. Morphological and morphometric characterisation of Onuf’s nucleus in the spinal cord of man. J Anat. 1997;191:201–213. doi: 10.1046/j.1469-7580.1997.19120201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF. Gonadal influences on sexual behavior in the male musk shrew (Suncus murinus) Horm. Behav. 1987;21:132–136. doi: 10.1016/0018-506x(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Bronson FH. The role of the ovary and adrenal gland in the sexual behavior of the musk shrew Suncus murinus. Biol Reprod. 1987;36:664–668. doi: 10.1095/biolreprod36.3.664. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Crews D. Hormonal correlates of sexual behavior in the female musk shrew (Suncus murinus): the role of estradiol. Physiol, Behav. 1988;26:76–86. doi: 10.1016/0031-9384(88)90338-1. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: firsts in androgen-dependent neural sex differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AM, Payne AP. Perinatal androgen administration and the maintenance of sexually dimorphic and nondimorphic lumbosacral motor neuron groups in female Albino Swiss rats. J Anat. 1991;177:47–53. [PMC free article] [PubMed] [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21(3):1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge K, Jennum P, Lokkegaard A, Werdelin L. Anal sphincter EMG in the diagnosis of parkinsonian syndromes. Acta Neurol Scand. 2010;121(3):198–203. doi: 10.1111/j.1600-0404.2009.01169.x. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Monks DA, Breedlove SM, Jordan CL. Androgen-sensitivity of somata and dendrites of spinal nucleus of the bulbocavernosus (SNB) motoneurons in male C57BL6J mice. Horm Behav. 2007;51:207–212. doi: 10.1016/j.yhbeh.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]