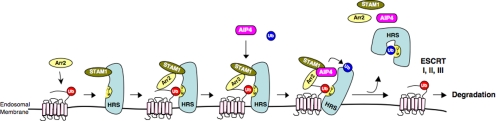
Figure 10.
Proposed mechanism for the role of the STAM-1/arrestin-2 complex in endosomal sorting of CXCR4. CXCR4 is ubiquitinated by the E3 ubiquitin ligase AIP4 at the plasma membrane, after which it is internalized onto early endosomes, although ubiquitination is not required for this process. Once on endosomes ubiquitinated CXCR4 is recognized by HRS, likely by an interaction involving the ubiquitin moiety (red) on CXCR4 and the UIM of HRS, and possibly via an interaction with arrestin-2. Arrestin-2 then interacts with STAM-1, which serves to recruit AIP4 culminating in the ubiquitination of HRS. We speculate that this triggers a conformational change in HRS induced by an interaction between the ubiquitin moiety (blue) and the internal UIM. CXCR4 is subsequently committed to downstream interactions with ESCRT-I-III, whereas arrestin-2, STAM-1, AIP4, and autoinhibited HRS are recycled such that another round of sorting can take place.