Fig. 3.
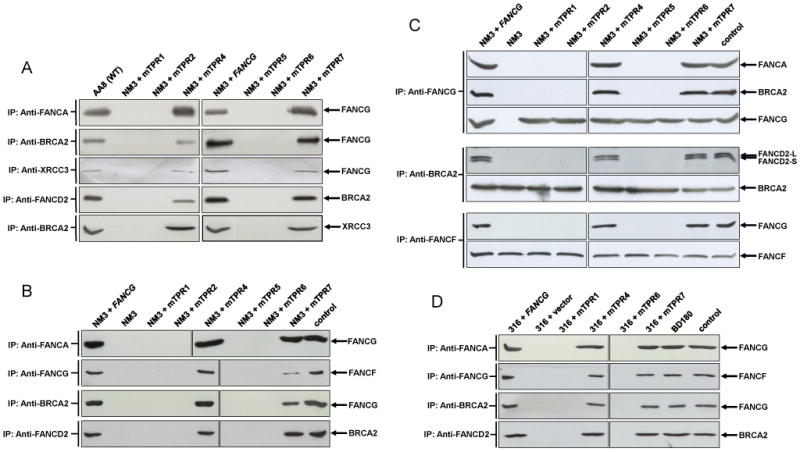
Interactions of TPR-mutated FANCG proteins in hamster NM3 cells (A, B and C) and human EUFA316 cells (D) as determined by co-immunoprecipitation analysis. Whole cell lysates from cultures treated with 50 nM MMC for 18 h were immunoprecipitated with the indicated anti-body and samples were loaded onto a SDS-PAGE gel. Following electrophoresis and membrane transfer, the blot was probed for the indicated protein. (A) The upper panel shows that mutations at TPR1, TPR2, TPR5 and TPR6 fail to support the interaction of FANCG with FANCA in NM3 cells. FANCG proteins with the same mutations were previously shown not to interact with FANCA in human FA-G EUFA316 cells (Blom et al. 2004), thereby confirming the suitability of the NM3 cell lines to analyse protein interactions in the D1-D2-G-X3 complex. The lower panel of (A) shows that FANCG mutated at TPR1, TPR2, TPR5 and TPR6 fails to co-precipitate with its direct binding partners BRCA2 and XRCC3 in the D1-D2-G-X3 complex and these same mutations fail to support the indirect protein interactions between BRCA2-FANCD2 and BRCA2-XRCC3. (B) Immunoprecipitation between FANCG-FANCF in NM3 cells and repeats for FANCA-FANCF, BRCA2-FANCG and BRCA2-FANCD2. Mutated TPR1, 2, 5 and 6 fail to support the interaction between FANCG-FANCF and appears to be reduced in NM3+mTPR7. The lane labelled input was loaded with1/50 of the protein extract used for immunoprecipitation in NM3+FANCG. (C) Reciprocal immunoprecipitations for FANCG-FANCA, FANCG-BRCA2, BRCA2-FANCD2 and FANCF-FANCG in NM3 cells. The lane labelled input was loaded with1/50 of the protein extract used for immunoprecipitation in NM3+FANCG. When immunoprecipitated with anti-FANCG, no interaction is detected between FANCG-FANCA and FANCG-BRCA2 in NM3, NM3+mTPR1, +mTPR2, +mTPR5 or +mTPR6, whilst blotting for FANCG acts as a control for the immunoprecipitation and indicates the expression of FANCG in these cell lines. Immunoprecipitation with anti-BRCA2 shows the interaction of BRCA2 with both isoforms of FANCD2 in NM3+FANCG, NM3+mTPR4 and +TPR7, with no interaction detected with either isoform of FANCD2 in NM3, NM3+mTPR1, +TPR2, +TPR5 or +TPR6. Immunoprecipitation with anti-FANCF confirms the lack of interaction between FANCF-FANCG in NM3, NM3+TPR1, +TPR2, +TPR5 and +TPR6 and interaction in NM3+FANCG and NM3+TPR4. No reduction in the interaction between FANCF-FANCG in NM3+TPR7 was evident in this experiment. (D) Protein interactions in selected human EUFA316 cells. Immunoprecipitations were the same as those shown for NM3 in Fig. 3B. The lane labelled input was loaded with1/50 of the protein extract used for immunoprecipitation in EUFA316+FANCG. Co-precipitation of FANCA-FANCG, FANCG-FANCF, BRCA2-FANCG and FANCD2-BRCA2 was observed in BD180 (wild type), EUFA316+FANCG, +mTPR4 and +mTPR7, whilst it was absent in EUFA316+vector, +TPR1 and +TPR6.
