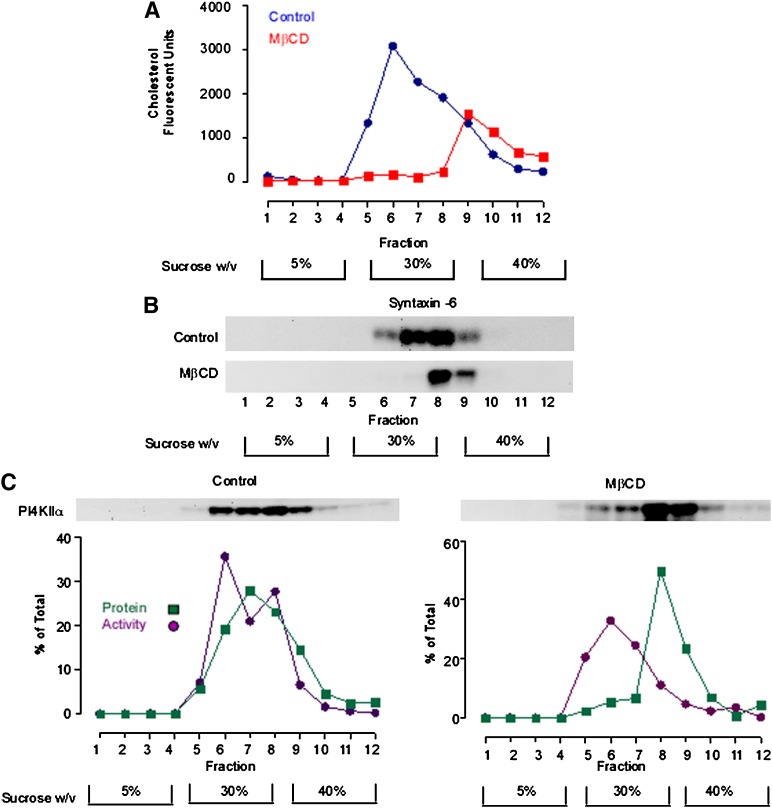
Fig. 2.
MβCD alters the steady-state compartmentation of PI4KIIα. A TGN-enriched membrane fraction was probe sonicated and subfractionated in a discontinuous sucrose density gradient. A: The cholesterol content of equal volume aliquots from each gradient fraction was determined using the Amplex Red cholesterol assay. B: Anti-syntaxin 6 Western blot demonstrating the gradient distribution of syntaxin-6 in control and sterol-depleted membrane fractions. C: Gradient distribution of PI4KIIα protein and activity. Equal volume samples from each density gradient fraction were Western blotted and probed with an anti-PI4KIIα antibody. PI4KIIα activity associated with each fraction was determined by solubilizing the membranes and measuring [32P]PI4P synthesis using a fixed concentration of micellar PI. PI4KIIα protein levels were determined by quantitative analysis of anti-PI4KIIα Western blots. Specific PI4KIIα activity associated with each fraction was calculated by dividing the rate of PI4P generation (phosphorimager units.min−1) by the amount of PI4KIIα protein present in each fraction as determined by Western blotting (arbitrary units). The results presented here are representative of three independent experiments. MβCD, methyl-β-cyclodextrin; PI4KII, type II phosphatidylinositol 4-kinase; PI4P, phosphatidylinositol 4-phosphate.