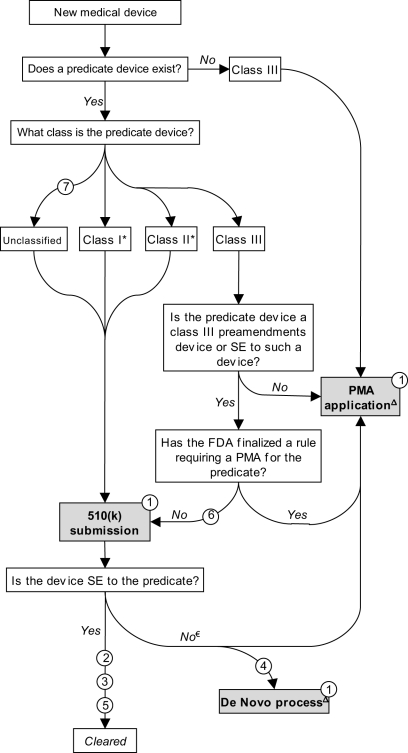
Figure 1. Schematic representation of medical device premarket review mechanisms.
Note: Issues listed in circles. Issue 8 does not appear in Figure 1. SE, substantially equivalent. * The 1997 FDAMA exempted most class I devices and a small number of class II devices from 510(k) requirements. € If determined to be not substantially equivalent, the sponsor may submit a PMA application. Alternatively, a sponsor may request evaluation under the de novo pathway (see text). Δ Post-decision scheme not illustrated.