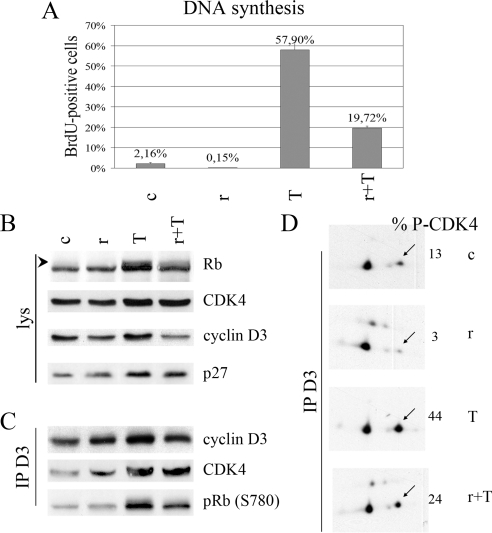
Figure 7.
mTORC1 activity is involved in cAMP-dependent cell cycle progression and CDK4 activation in dog thyrocytes in primary culture. Dog thyrocytes cultured with 1 μg/ml insulin were stimulated without (c) or with 1 mU/ml TSH (T) for 48 h (panel A) or 20 h (panels B–D) in the presence or not of 40 nm rapamycin (r). A, Rapamycin inhibits TSH-induced DNA synthesis. BrdU was present during the last 24 h. The percentage of BrdU-positive nuclei was counted at the microscope (mean + range from duplicate dishes). B, Rapamycin inhibits TSH-stimulated Rb phosphorylation. Rb, CDK4, cyclin D3, and p27 were immunodetected from whole-cell lysates (lys). The arrowhead indicates phosphorylated forms of Rb. C, Rapamycin inhibits TSH-stimulated cyclin D3-CDK4 activity. Cell lysates were immunoprecipitated (IP) with anticyclin D3 (D3) antibodies, assayed for Rb-kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4, and the in vitro S780 phosphorylation of the Rb fragment [pRb (S780)] were detected using specific antibodies. D, Rapamycin inhibits basal and TSH-stimulated activating phosphorylation of CDK4. Cyclin D3-CDK4 complexes were immunoprecipitated (IP) with cyclin D3 antibodies (D3). The immune complexes were separated by 2-dimensional gel electrophoresis, and CDK4 was immunodetected. The percentage of the T172-phosphorylated form of CDK4 (arrows) vs. total CDK4 is indicated (% P-CDK4).