αA Crystallins are induced by toll-like receptors in the retina during EAU.
Abstract
Purpose.
Previous studies indicate that the upregulation of αA crystallin prevents photoreceptor mitochondrial oxidative stress–mediated apoptosis in experimental autoimmune uveitis (EAU). In this study, the role of TLR4 was investigated in the upregulation of αA crystallin in the retinas of animals with EAU.
Methods.
TLR4−/−, iNOS−/−, TNF-α−/−, MyD88−/−, wild-type (WT) control (C57BL/6), and nude mice (B6.Cg-Foxn1nu) were immunized with IRBP mixed with complete Freund's adjuvant; eyes were enucleated on day 7 after immunization. Real-time polymerase chain reaction was first used to detect upregulated inflammatory cytokines and αA crystallin in retinas with EAU; confirmed with Western blot analysis, and the site of upregulation was localized by immunohistochemistry. Oxidative stress was localized using 8-OHdG, and TUNEL staining was used to detect apoptosis.
Results.
In early EAU, increased expression of TNF-α, iNOS, and αA crystallin genes were detected in the retinas of WT mice, whereas such upregulation was absent in TLR4-deficient mice (P < 0.001). αA Crystallin was not elevated in MyD88−/−, TNF-α−/−, and iNOS−/− mice with EAU. Immunostaining revealed TNF-α, iNOS, and αA crystallin localization in the photoreceptor inner segments and outer plexiform layer in the WT controls with EAU; but such staining was absent in TLR4-deficient mice with EAU. 8-OHdG staining showed oxidative stress in the photoreceptors in WT mice with EAU and there was no apoptosis.
Conclusions.
TLR4 plays an important role in the upregulation of αA crystallin through the interaction of MyD88 and the subsequent generation of TNF-α and iNOS in the EAU retina. Such crystallin upregulation may prevent oxidative stress–mediated apoptosis of photoreceptors in uveitis.
Uveitis constitutes a diverse group of entities in which oxygen metabolites play a role in retinal photoreceptor apoptosis and amplification of inflammation, leading to significant vision loss and other ocular morbidities.1 This intraocular inflammatory disease is a leading cause of blindness, primarily because of retinal photoreceptor degeneration.2 A robust animal model of uveitis that closely resembles human uveitis is experimental autoimmune uveitis (EAU).3 Blood-borne activated macrophages are major effectors of retinal tissue damage observed during EAU.4–6 However, recent studies show that oxidative stress, peroxynitrate-mediated nitration of photoreceptor mitochondrial proteins, including cytochrome c, occurs in early EAU before the leukocytes infiltrate the retina.7–9 These observations suggest that mitochondrial oxidative stress could be the initial pathologic effector event in EAU-related photoreceptor damage. However, previous studies largely ignored the early events in the inflammatory process that lead to photoreceptor cell apoptosis and regulatory or protective mechanisms in the retina.2,5,6,9–14
A recent study revealed that αA-crystallin is highly upregulated in the retina during early EAU; this upregulation is localized primarily in the photoreceptor inner segments, the site of mitochondrial oxidative stress in early EAU, and the photoreceptors preferentially use αA-crystallin to abrogate mitochondrial oxidative stress-mediated apoptosis.15 Although the pathway leading to αA-crystallin upregulation has not been studied thoroughly in EAU, the upregulation of crystallins during early EAU suggests a role for the innate immune response.
Toll-like receptors (TLRs), a group of transmembrane proteins, play a crucial role in innate immune response16 and in the generation of TNF-α.17 Among the various TLRs, in vitro studies suggest that activation of TLR4, the first-characterized mammalian Toll,18 can cause mitochondrial oxidative stress in central nervous system neurons and hepatocytes.19 In addition, TLR4 has been genetically identified as an essential and nonredundant component of the lipopolysaccharides receptor signaling complex that controls innate immune response in vivo.20–22
The present study was designed to investigate the role of TLR4 in the initiation of αA-crystallin upregulation through the interaction of its adaptor protein MyD88 and other molecules in the signal transduction pathway in the retinas of animals with early EAU and its distribution at the sites of oxidative stress.
Materials and Methods
Induction and Detection of EAU in the Retina
Animal care and use was in compliance with institutional guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. EAU was induced in 8-week-old C57BL/6 (wild-type [WT]), B6.Cg-Foxn1nu (nude mice), TLR4−/−, iNOS−/−, TNF-α−/− mice (Jackson Laboratory, Bar Harbor, ME), and MYD88−/− mice (kindly provided by Deming Sun, Doheny Eye Institute, Los Angeles, CA). Induction of EAU was made by immunizing the mice with 150 μg IRBP (1–20; GPTHLFQPSLVLDMAKVLLD) in PBS-emulsified 1:1 vol/vol in complete Freund's adjuvant (CFA) supplemented with MTB to 2.5 mg/mL. A total of 300 μL emulsion was injected subcutaneously, divided between the base of the tail and both thighs. Pertussis toxin (0.5 μg/mouse) in PBS was given by intraperitoneal injection concurrently with immunization.23 Nonimmunized WT mice, nonimmunized TLR4−/−, and WT mice injected with CFA and pertussis toxin alone served as controls.
Detection of Upregulated Genes by Real-time PCR in Early EAU
Six mice induced with EAU from each group of nude, TLR4−/−, iNOS−/−, TNF-α−/−, MyD88−/−, and WT mice, along with nonimmunized WT and TLR4−/− mice, were euthanatized on day 7 after immunization (early EAU). Eyes from each group were enucleated, and the retinas were dissected without lens material contamination. RNA was extracted using reagent (Trizol; Invitrogen, Carlsbad, CA) from each group of retinas. The cDNA template was generated using the reverse transcription kit (Promega, Madison, WI). Real-time polymerase chain reaction (PCR) was performed to detect gene expression levels of TNF-α, iNOS, and αA crystallin. Each 25-μL PCR reaction mixture contained a master mix (SYBR Green I; Bio-Rad Laboratories, Hercules, CA); 0.5 μM gene-specific primers for TNF-α, iNOS, and αA; and the cDNA template. In the quantification analysis, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalizing gene (Table 1).
Table 1.
Sequence of Primers Specific for TNF-α, iNOS, and αA Crystallin Used for Real-time PCR Analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | CTACTCCCAGGTTCTCTTCAA | GCAGAGAGGAGGTTGACTTTC |
| iNOS | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG |
| αA crystallin | CAGGGACCACAGCAAAGAGT | GCCACTTTCCAGGAAGACAG |
| GAPDH | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA |
PCR reactions for each gene in each experiment were performed in triplicate on each cDNA template, along with triplicate reactions of the housekeeping gene GAPDH. Dissociation melting curve analysis was used to check the specificity of PCR amplification products. The threshold cycle (Ct) difference between the experimental and control groups, for each gene in each tissue, was calculated and normalized to GAPDH, and the increase (x-fold) in mRNA expression was determined by the 2-ΔΔCt method.24
Statistical analysis of ΔΔCt was performed with ANOVA followed by Tukey-Kramer multiple comparison test for three independent samples, with significance set as P < 0.05 using statistical software (InStat; GraphPad, San Diego, CA). The experiments were performed in triplicate.
Western Blot Analysis of TNF-α, iNOS, and αA Crystallin
On day 7 after immunization, retinas of five EAU mice each of nude, WT, TLR4−/−, iNOS−/−, TNF-α−/−, five WT mice injected with pertussis toxin and CFA alone, and five nonimmunized WT (control) mice were dissected, homogenized, and lysed in protein extraction buffer (M-PER; Thermo Scientific, Waltham, MA) containing protease inhibitors (Calbiochem, San Diego, CA). The homogenates were then sonicated for 30 seconds and centrifuged at 13,000 rpm for 20 minutes at 4°C. Protein quantification of the supernatant was determined using bovine serum albumin as the standard (Bio-Rad Laboratories). Equal amounts of protein samples were loaded and run on SDS-PAGE (15% Tris-HCl polyacrylamide ready gels [Bio-Rad Laboratories]; to detect iNOS, 7.5% Tris-HCl polyacrylamide ready gels were used (Bio-Rad Laboratories). After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories) using a transblot semidry system. The membranes were blocked using 5% skim milk and then probed with a polyclonal anti–αA-crystallin (Stressgen, Ann Arbor, MI) at a 1:2500 dilution overnight at 4°C. Similarly, iNOS and TNF-α were detected by probing the membranes with polyclonal anti-iNOS (BD Transduction Laboratories, San Jose, CA) and monoclonal TNF-α (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, with iNOS at a 1:2000 dilution and TNF-α at a 1:500 dilution. After incubation for 45 minutes with the secondary antibody tagged with horseradish peroxidase (anti-mouse or anti-rabbit, depending on the primary antibody used; Santa Cruz Biotechnology), signals were detected by chemiluminescence system (Thermo Scientific). Equal protein loading of retinal lysates from each group of animals was confirmed by reprobing blots with a monoclonal antibody to β-actin.
Localization of αA Crystallin, NFκB, iNOS, and TNF-α in the Early EAU Retina
Seven-micrometer cryosections were obtained from retinas of five early EAU WT and TLR4−/− mice and five nonimmunized WT and TLR4−/− control mice. To localize αA crystallin, iNOS, nuclear factor κB (NFκB), and TNF-α retinal cryosections were probed at a 1:00 dilution with a polyclonal anti–αA-crystallin (Stressgen), monoclonal anti-iNOS (BD Transduction Laboratories), polyclonal anti-NFκB (Santa Cruz Biotechnology), and monoclonal TNF-α (Santa Cruz Biotechnology). The secondaries used (1:200 dilution) were either Cy-2–conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or Texas Red dye–conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.), depending on the primary antibodies used. All sections were viewed by confocal microscopy (Carl Zeiss, Oberkochen, Germany). Isotype controls and PBS-replaced primary antibody were used as the negative controls. The experiments were performed in triplicate.
Localization of Oxidative DNA Damage and Detection of Apoptotic Cells in the Early EAU Retina
Seven-micrometer cryosections were obtained from retinas of five WT EAU, TLR4−/− EAU, nonimmunized WT, and nonimmunized TLR4−/− mice. 8-Hydroxy-deguanosine (8-OHdG) was detected using polyclonal anti–8-OHdG (Chemicon, Temecula, CA) at 1:50 dilution and Texas Red dye–conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc.) at 1:200 dilution. All sections were viewed by confocal microscopy. Isotype controls and PBS-replaced primary antibody were used as the negative controls. Experiments were performed in triplicate. The TUNEL procedure was performed with an apoptosis detection kit (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Label solution was used in place of the enzyme solution as the negative control, and positive control slides were treated (DNase I recombinant; Roche Diagnostics). Staining was performed in triplicate and was viewed with confocal microscopy.
Results
Detection of Upregulated Genes by Real-time PCR in Early EAU
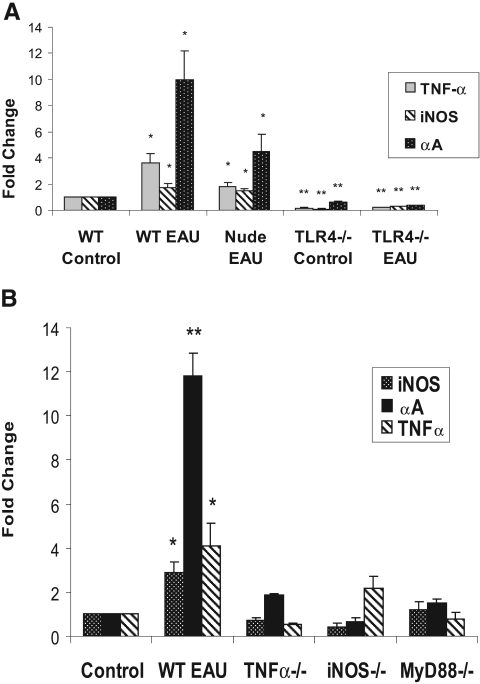
In early EAU retinas, αA crystallin increased 10-fold in WT EAU mice (P < 0.05) and 4.5-fold in nude mice with EAU (P < 0.05) compared with nonimmunized WT controls. TLR4−/− mice with EAU, on the other hand, showed a 2.7-fold decrease (P < 0.001) compared with WT controls. There was also a corresponding increase in TNF-α in WT EAU and nude EAU mice (3.6-fold and 1.8-fold, respectively; P < 0.05) and a corresponding decrease in TNF-α in TLR4−/− mice with EAU (5.0-fold; P < 0.001) compared with WT controls. Similarly, iNOS increased 1.8-fold in WT EAU (P < 0.05), increased 1.5-fold in nude EAU (P < 0.05), and decreased 3.2-fold in TLR4−/− EAU (P < 0.001) mice compared with nonimmunized WT controls (Fig. 1A). Inthe iNOS−/− and the TNF-α−/− EAU mice, there was no significant increase in αA Crystallin expression compared with WT control mice (P > 0.05). Similarly, in the TNF-α−/− mice, iNOS was not upregulated; in the iNOS−/− mice, there was no significant elevation of TNF-α expression during early EAU (P > 0.05). TNF-α, iNOS, and αA crystallin were also not upregulated in MyD88−/− mice in early EAU (P > 0.05) (Fig. 1B).
Figure 1.
(A) Marked upregulation of TNF-α, iNOS, and αA crystallin were found in WT mice with EAU retinas compared with nude mice with EAU, TLR4−/− mice with EAU, and nonimmunized controls. Upregulation of TNF-α, iNOS, and αA crystallin was also found in nude mice with EAU compared with TLR4−/− mice with EAU and nonimmunized controls. TNF-α, iNOS, and αA crystallin were all downregulated in TLR4−/− mice with EAU compared with nonimmunized WT controls. (B) TNF-α, iNOS, and αA crystallin were not upregulated in MyD88−/− mice. iNOS and αA crystallin were not elevated in TNF-α−/− mice. Similarly, there was no upregulation of αA crystallin or TNF-α in iNOS−/− mice. Gene expression of TNF-α, iNOS, and αA crystallin during early EAU in C57BL/6 (WT), B6.Cg-Foxn1nu (nude), TLR4−/−, MyD88−/−, TNF-α−/−, and iNOS−/− mice by real-time PCR analysis using gene-specific primers and normalized to GAPDH. The relative multiple of change in mRNA expression was determined using the 2-ΔΔCt method. *P < 0.05; **P < 0.001.
Western Blot Analysis of TNF-α, iNOS, and αA Crystallin
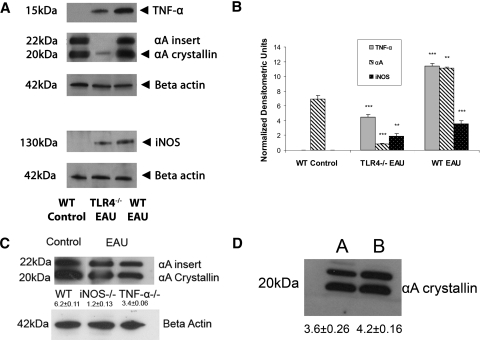
In the retinal homogenates of WT EAU animals and WT control animals, a prominent band of αA crystallin was detected, as expected at 20 kDa (Figs. 2A, 2C, 2D). A second band was seen at 22 kDa and indicated an αA insert, as previously published15). In early EAU retinas of WT mice, there was a 1.6-fold increase in the αA crystallin protein level in the retina compared with the control groups (Fig. 2B). TLR4−/− mice with EAU showed a ninefold decrease in αA crystallin compared with WT controls. Densitometry measurements also show a threefold decrease in TNF-α, a 14-fold decrease in αA crystallin, and a twofold decrease in iNOS protein in TLR4−/− mice with early EAU compared with WT EAU mice (Fig. 2B). However, there was no significant increase in the levels of αA crystallin in the iNOS−/− or in the TNF-α−/− mice in early EAU compared with the WT controls (Fig. 2C). In the WT mice injected with pertussis toxin and CFA, there was no significant increase in αA crystallin expression compared to the WT control (Fig. 2D). For the upregulation of αA crystallin in early EAU, real-time PCR showed a 10-fold increase compared with nonimmunized WT controls, whereas in Western blot analysis, the increase in protein was only 1.6-fold. The reason for this discrepancy could be multifactorial. The upregulation of gene and protein in vivo does not always have a direct correlation, in part because of the low stability of transcripts generated and the low translational efficiency. In addition, for low molecular weight proteins (such as αA crystallin), the run-off from SDS-PAGE gel could become apparent.15
Figure 2.
During early EAU, TNF-α, αA crystallin, and iNOS were significantly upregulated compared with both nonimmunized WT control and TLR4−/− mice with EAU (A, B). There was no upregulation of αA crystallin in iNOS−/− or in TNF-α−/− mice in early EAU (C). There was no significant increase in the expression of αA crystallin in the mice treated with CFA and pertussis toxin (D; A, WT control; B, WT injected with pertussis toxin). Equal amounts of total retinal proteins from day 7 WT control, WT EAU, TLR4−/−, TNF-α−/−, and iNOS−/− mice with EAU and WT mice injected with CFA and pertussis toxin were separated on a 15% SDS-polyacrylamide gel (7.5% gel for iNOS). Protein bands were transferred to a nitrocellulose membrane and probed with monoclonal TNF-α, polyclonal anti–αA-crystallin, and polyclonal anti-iNOS as the primary antibodies and with corresponding secondary antibodies tagged with horseradish peroxidase. TNF-α was detected at the molecular mass indicated (∼15 kDa), αA crystallin was detected at ∼20 kDa protein, and iNOS was detected at ∼130 kDa protein. Equal protein loading was confirmed by reprobing blots with monoclonal antibody to β-actin. There was a significant decrease in αA crystallin in the day 7 EAU retina of TLR4−/− mice compared with the WT control retina. (B) Densitometry measurements show a threefold decrease in TNF-α, a 13-fold decrease in αA crystallin, and a twofold decrease in iNOS protein in TLR4−/− mice with early EAU compared with WT EAU mice. There was also a ninefold decrease in αA crystallin in TLR4−/− mice with early EAU compared with WT control. *P < 0.05; **P < 0.001; ***P < 0.0001.
Localization of αA Crystallin, NFκB, iNOS, and TNF-α in the Early EAU Retina
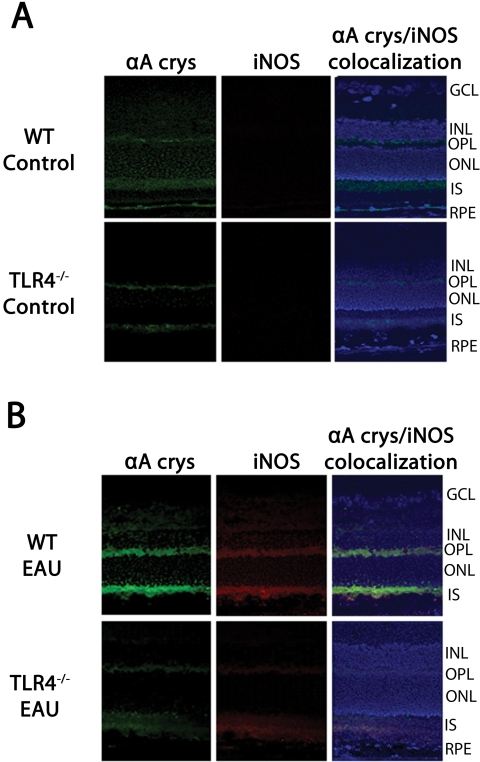
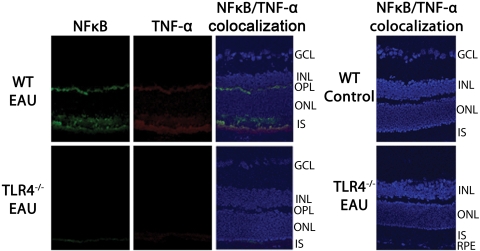
Immunostaining of nonimmunized C57BL/6 (WT) mice showed αA crystallin staining in the outer plexiform layer and the photoreceptor inner segments. Nonimmunized TLR4−/− mice also showed very weak staining in these retinal layers. In both nonimmunized WT and TLR4−/− mice, there was no detectable amount of iNOS staining (Fig. 3A). However, immunostaining of WT mice during early EAU revealed intense staining of iNOS and αA crystallin in the photoreceptor inner segments and outer plexiform layer, whereas only minimal staining was present in the inner segments and outer plexiform layer in TLR4−/− mice with EAU (Fig. 3B). Nonimmunized WT and nonimmunized TLR4−/− mice showed no NFκB or TNF-α staining in the retina. During early EAU in WT mice, NFκB and TNF-α were localized to the inner segments of the photoreceptors and the outer plexiform layers; such staining was absent in TLR4−/− mice with EAU (Fig. 4).
Figure 3.
Immunofluorescence localization of αA crystallin and iNOS in the retina. Tissues were labeled using polyclonal antibody against αA-crystallin and monoclonal anti-iNOS (primary antibodies) and against Cy-2–conjugated donkey anti-rabbit IgG and Texas Red dye–conjugated donkey anti-mouse IgG, respectively (secondary antibodies). (A) Immunostaining of nonimmunized C57BL/6 (WT) mice showed αA crystallin localized in the outer plexiform layer and the photoreceptor inner segments. Nonimmunized TLR4−/− mice also showed very weak staining in these retinal layers. In both nonimmunized WT and TLR4−/− mice, there was no detectable amount of iNOS staining. (B) Immunostaining of WT mice EAU day 7 after immunization revealed intense staining of iNOS and αA crystallin in the photoreceptor inner segments and outer plexiform layer; however, minimal staining was present in the inner segments and outer plexiform layer in TLR4−/− mice with EAU.
Figure 4.
Immunofluorescence localization of NFκB and TNF-α in the retinas of mice with EAU day 7 after immunization. Tissues were labeled using polyclonal anti-NFκB and monoclonal antibody against TNF-α (primary antibodies) along with Cy-2–conjugated donkey anti–rabbit IgG and Texas Red dye–conjugated donkey anti–mouse IgG, respectively (secondary antibodies). Nonimmunized WT and nonimmunized TLR4−/− mice showed no NFκB or TNF-α staining in the retina. During early EAU, however, WT mice showed NFκB and TNF-α staining in the inner segments of the photoreceptors and the outer plexiform layers, whereas such staining was absent in TLR4−/− mice.
Localization of Oxidative DNA Damage and Detection of Apoptotic Cells in the Early EAU Retina
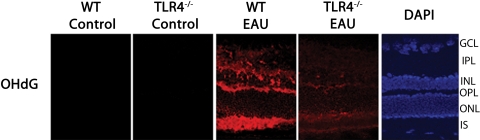
No 8-OHdG staining was present in the nonimmunized WT and TLR4−/− mice. However, in the WT mice with EAU, intense OHdG staining was detected in the inner segment of the photoreceptor layer, inner nuclear layer, outer plexiform layer, and inner plexiform layer; such staining was markedly reduced in the inner segments and outer plexiform layer and was absent in the remainder of the retina in the TLR4−/− mice during early EAU (Fig. 5). TUNEL-positive cells were not detected in WT and TLR4−/− during early EAU. Neither the negative control nor the nonimmunized WT control showed TUNEL-positive cells. In contrast, numerous apoptotic cells were seen in positive control retinas treated with DNase I (Fig. 6).
Figure 5.
Immunofluorescence localization of 8-OHdG in the retina. Tissues were labeled using polyclonal antibody against 8-OHdG (primary antibody) and Texas Red dye–conjugated donkey anti–goat IgG (secondary antibody). No 8-OHdG staining was present in the nonimmunized WT or TLR4−/− mice. In the WT mice with EAU (day 7 after immunization), intense OHdG staining was detected in the inner segment of the photoreceptor layer, inner nuclear layer, outer plexiform layer, and inner plexiform layer; such staining was markedly reduced in the inner segments and outer plexiform layer and was absent in the remainder of the retina in the TLR4−/− mice during early EAU.
Figure 6.
Both the negative control and the nonimmunized WT control showed no apoptosis. TUNEL-positive cells were also not detected in WT and TLR4−/− during early EAU. In contrast, numerous apoptotic cells were seen in positive control retinas treated with DNase I. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; IPL, inner plexiform layer.
Discussion
In our study, real-time PCR analysis revealed that during early EAU, before leukocyte infiltration of the uvea and the retina, there is increased expression of TNF-α and iNOS in nude mice (B6.Cg-Foxn1nu) compared with nonimmunized controls (Fig. 1). Given that nude mice are deficient in T cells,25 the presence of inflammatory cytokines during early EAU suggests a role of innate immunity in the expression of these cytokines. This role was further confirmed using real-time PCR on WT mice with EAU and TLR4 knockout mice with EAU; TNF-α and iNOS were significantly upregulated in WT mice with EAU compared with nonimmunized controls and nude mice with EAU. However, these genes were downregulated in TLR4-deficient mice compared with both nonimmunized and WT mice with EAU (Fig 1A); such findings suggest that TLR4 plays a role in the generation of the proinflammatory cytokines, and such cytokines are known to initiate mitochondrial oxidative stress.8 A similar finding was reported by Suliman et al.,19 who demonstrated that TLR4−/− mice showed negligible TNF-α responses when compared with mice that expressed TLR4 normally. TLR4 deficiency was shown to attenuate iNOS gene expression. In our study, TNF-α, and iNOS were not upregulated in MyD88−/− mice. Moreover TNF-α was not elevated in iNOS−/− mice and iNOS was not upregulated in TNF-α−/− mice with EAU (Fig. 1B). Activation of the innate immune system through TLR results in its interaction with its adaptor protein MyD88 and initiates a complex signaling transduction that activates NFκB, leading to the generation of proinflammatory cytokines such as TNF-α.17,18,26 NFκB is found in the cytoplasm in the normal condition, where it is complexed with an inhibitory protein (IκB). It can be activated by a variety of stimuli, including TLRs and cytokines.27 TNF-α, in turn, subsequently induces the expression of iNOS, which is known to cause mitochondrial oxidative stress.8
In mammals there are at least 10 distinct TLR family members.28,29 Among these receptors, TLR4 is known to induce mitochondrial oxidative stress in the liver.19 Similar mitochondrial oxidative stress is seen in the photoreceptors in early EAU.30 Our present results show that during early EAU, there was activated NFκB with upregulation of TNF-α and iNOS (Figs. 3, 4), inducing photoreceptor mitochondrial oxidative stress. Such stress was abrogated in the absence of TLR4, as observed in the TLR4 knockout (Fig. 5).
Interestingly, TLR4 plays a major role in the modulation of αA crystallin expression because the expression of this crystallin was enhanced in EAU in animals with intact TLR4. Such a phenomenon was minimal or absent in the TLR4−/− animals with EAU (Fig. 3B). mRNA expression of αA crystallin was confirmed by the quantification of protein levels by immunoblot analysis in the nonimmunized WT control, WT mice with EAU, and TLR4 knockout mice with EAU. The immunoblot showed two protein bands, one with a molecular mass of 20 kDa, corresponding to αA crystallin, and the second with a molecular mass of 22 kDa (αA insert crystallin), similar to our previously published findings.15 The αA crystallin insert is an alternatively spliced product that has a C terminus identical to that of the αA crystallin; hence, the affinity of the antibody used for detection is the same for both proteins. These two bands have been observed before both in mouse eyes15 and in rodent lenses.31–33 The WT mice with EAU showed increased protein levels of αA crystallin, as reported earlier.15 However, TLR4-deficient mice showed only mild staining at 20 kDa, reaffirming our hypothesis that TLR4 is essential in the upregulation of αA crystallin. The protein level was lower in the TLR4−/− mice than in normal controls, indicating that there might be a global defect of αA crystallin in these mice lacking TLR4. Further sequential studies on expression levels of this protein in TLR4−/− mice during different stages of EAU would help us to address this issue. Moreover, there was no increase in the mRNA expression of αA crystallin in iNOS−/− or in TNF-α−/− mice during early EAU (Fig. 1B). Such findings also suggest that oxidative stress induced by TNF-α and iNOS could be responsible for the upregulation of αA crystallin. The absence of αA crystallin elevation in MyD88−/− mice during early EAU indicate that the adaptor protein MyD88 is essential for the signal transduction leading to the activation of NFκB, TNF-α, and iNOS and the subsequent induction of αA crystallin.
The protein level of αA crystallin in iNOS−/− and TNF-α−/− mice with EAU was also confirmed by immunoblot. The results showed no increase in its protein level compared with control mice with EAU (Fig. 2C). This clearly indicated that both TNF-α and iNOS are responsible for increased expression of αA crystallin in early EAU. iNOS−/− mice have been demonstrated to show an absence of oxidative stress and apoptosis.34 Previous reports have shown that pertussis toxin acts as a ligand for different Toll-like receptors.35 However, in our study, there was no significant increase in the protein level of αA crystallin in the mice injected with CFA and pertussis toxin (Fig. 2D).
WT mice with early EAU demonstrated intense immunostaining for the crystallin in the outer retina, particularly in the inner segments of the photoreceptors, and moderate staining in the outer plexiform layer and the inner nuclear layer. In contrast, TLR4-deficient mice with early EAU and nonimmunized WT and TLR4−/− controls showed an absence of such upregulation in the inner and outer segments of the photoreceptors (Figs. 3A, 3B). This corresponds with previous findings that report that, under normal conditions, αA crystallin has little or no staining in the inner and outer segments of the photoreceptors36 and that in EAU retinas, photoreceptor cells are the site of oxidative damage.7,8 Given that TLR4-deficient mice with EAU did not show intense staining of the inner segments, these data support our hypothesis that TLR4 could play a role in the upregulation of αA crystallin; the data also suggest the involvement of αA crystallin in the prevention of photoreceptor cell apoptosis in EAU.12
Our study also revealed that the presence of TLR4 during early EAU is necessary for oxidative damage in DNA. The study of mitochondrial DNA offers a reliable biomarker of oxidative stress in tissue rich in mitochondria, such as the photoreceptor inner segments of the retina. During early EAU, reactive oxidants and peroxynitrite are also localized in the photoreceptor inner segment mitochondria.9 A recent study in our laboratory showed that mitochondrial oxidative DNA damage occurs early in the EAU retina, whereas nuclear DNA damage occurs later, suggesting the retinal damage may begin early from photoreceptor mitochondrial oxidative stress rather than from macrophage-derived noxious agents.37 8-OHdG is an oxidized form of deoxyguanosine, and the detection of 8-OHdG has been successfully used as a reliable marker of DNA oxidation damage. In oxidative stress, 8-OHdG levels increase preferentially in mitochondria as a result of the single-stranded nature of mitochondrial DNA, which makes it more susceptible to damage.38 In WT mice with EAU, intense OHdG staining was detected in the inner segments of the photoreceptor layer, inner nuclear layer, outer plexiform layer, and inner plexiform layer. In early EAU, mice deficient in TLR4 showed only very mild staining in the inner segments and outer plexiform layers, and staining was absent in the remainder of the retina (Fig. 5). Thus, there is substantial mitochondrial DNA damage in WT mice with EAU that is significantly reduced in TLR4 knockout mice.
The intense OHdG staining we found in the photoreceptor inner segments of WT mice with EAU reinforces previous findings indicating that in EAU retinas, photoreceptor cells are the site of oxidative damage.7,8 TNF-α is known to cause tissue damage by generating reactive oxygen species, including the upregulation of nitric oxide synthase (iNOS),39 and such upregulation of iNOS causes mitochondrial oxidative stress.40 iNOS-mediated oxidative stress results in nitration of the photoreceptor mitochondrial protein, cytochrome c, which is released into the cytosol.8,9 Such cytochrome c release is known to cause apoptosis, but apoptosis is not a feature of early EAU, suggesting the presence of a protective mechanism, such as αA crystallin upregulation, in the photoreceptors that prevents photoreceptor apoptosis in early EAU.15 Similarly, in the present study, apoptotic cells were not detected in the WT or in the TLR4 knockout mice during early EAU (Fig. 6).
We also found that during early EAU, αA crystallin was localized primarily in the photoreceptor inner segments, the site of oxidative stress; this suggests that photoreceptors may use αA crystallin to prevent oxidative stress-mediated damage.15
It is important to note that though the presence of TLR4 is necessary for significant amounts of TNF-α, iNOS, and αA crystallin upregulation, it is not the only pathway; other TLRs may also play a role in the induction of these gene products during early EAU because mild upregulation of these gene products and mild DNA oxygen damage still occurs when TLR4 is not present. A similar observation was reported by Su et al.,23 where the single TLR gene KO mice do not point to a unique role of these receptors in the induction of EAU. However, this observation, along with our findings, does not negate the possibility that TLR4 signaling plays a critical role in the induction of TNF-α, iNOS, and αA crystallin. Double and triple TLR knockouts may have to be generated for study of the roles of different TLRs in the induction of autoimmune diseases such as EAU. Nevertheless, this study shows that the absence of TLR4 during early EAU affects the upregulation of TNF-α, iNOS, and αA crystallin.
Addressing the functional significance of TLR4 in EAU and the important role TLR4 plays in inducing αA crystallin expression will enhance our understanding of both the EAU pathway and the importance of crystallin upregulation in neuronal inflammation as a protective molecule.
Footnotes
Supported in part by National Institutes of Health Grants EY017347 and EY03040 and by a grant from Research to Prevent Blindness, Inc., New York, NY.
Disclosure: S. Saraswathy, None; A.M. Nguyen, None; N.A. Rao, None
References
- 1.Arocker-Mettinger E, Grabner G. Uveitis: a status assessment. Fortschr Ophthalmol 1990;87(suppl):S110–S117 [PubMed] [Google Scholar]
- 2.Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol 2002;21:273–289 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med 2004;102:395–419 [DOI] [PubMed] [Google Scholar]
- 4.Rao NA. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc 1990;88:797–850 [PMC free article] [PubMed] [Google Scholar]
- 5.Forrester JV, Huitinga I, Lumsden L, Dijkstra CD. Marrow-derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr Eye Res 1998;17:426–437 [DOI] [PubMed] [Google Scholar]
- 6.Hoey S, Grabowski PS, Ralston SH, Forrester JV, Liversidge J. Nitric oxide accelerates the onset and increases the severity of experimental autoimmune uveoretinitis through an IFN-γ-dependent mechanism. J Immunol 1997;159:5132–5142 [PubMed] [Google Scholar]
- 7.Wu GS, Zhang J, Rao NA. Differential expression of nitric oxide synthase in experimental uveoretinitis. Invest Ophthalmol Vis Sci 1999;40:1899–1905 [PubMed] [Google Scholar]
- 8.Rajendram R, Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol 2007;92:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu GS, Lee TD, Moore RE, Rao NA. Photoreceptor mitochondrial tyrosine nitration in experimental uveitis. Invest Ophthalmol Vis Sci 2005;46:2271–2281 [DOI] [PubMed] [Google Scholar]
- 10.Morimoto RI. Stress, aging, and neurodegenerative disease. N Engl J Med 2006;355:2254–2255 [DOI] [PubMed] [Google Scholar]
- 11.Forrester JV, Liversidge J, Dua HS, Towler H, McMenamin PG. Comparison of clinical and experimental uveitis. Curr Eye Res 1990;9:75–84 [DOI] [PubMed] [Google Scholar]
- 12.Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res 2000;19:41–68 [DOI] [PubMed] [Google Scholar]
- 13.Rao NA, Kimoto T, Zamir E, et al. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci 2003;44:22–31 [DOI] [PubMed] [Google Scholar]
- 14.Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol 2002;21:197–208 [DOI] [PubMed] [Google Scholar]
- 15.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina specific expression of the small heat shock protein, alpha A crystallin is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci 2008;49:1161–1171 [DOI] [PubMed] [Google Scholar]
- 16.Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X. Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor (TLR)-4 and PI3K/Akt. Cell Biol Int 2009;33:665–674 [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy 2002;2:181–191 [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosphila Toll protein signals activation of adaptive immunity. Nature 1997;388:394–397 [DOI] [PubMed] [Google Scholar]
- 19.Suliman HB, Welly-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J 2005;19:1531–1533 [DOI] [PubMed] [Google Scholar]
- 20.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in the TLR4 gene. Science 1998;282:2085–2088 [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 1999;11:443–451 [DOI] [PubMed] [Google Scholar]
- 22.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-κB through Toll-like receptor 4 (TLR4) in cultured human dermal endothelial cells: differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem 2000;275:11058. [DOI] [PubMed] [Google Scholar]
- 23.Su SB, Silver PB, Grajewski RS, et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol 2005;175:6303–6310 [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmitten TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Balciunaite G, Keller MP, Balciunaite E, et al. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol 2002;3:1102–1108 [DOI] [PubMed] [Google Scholar]
- 26.Thoma-Uszynski S, Stenger S, Takeuchi O. Induction of direct antimicrobial activity through mammalian Toll-like receptor. Science 2001;291:1544–1547 [DOI] [PubMed] [Google Scholar]
- 27.Dordevic G, Matusan-llijas K, Sinozic E, et al. Relationship between vascular endothelial growth factor and nuclear factor-κB in renal cell tumors. Croatian Med J 2008;49.5:608–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145 [DOI] [PubMed] [Google Scholar]
- 29.Zuany-Amorim C, Hastewell J, Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat Rev Drug Discov 2002;3:216–227 [DOI] [PubMed] [Google Scholar]
- 30.Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in experimental autoimmune uveitis. Ophthalmic Res 2008;40:160–164 [DOI] [PubMed] [Google Scholar]
- 31.De Jong WW, Cohen LH, Leunissen JA, Zweers A. Internally elongated rodent α-crystallin A chain: resulting from incomplete RNA splicing. Biochem Biophys Res Commun 1980;96:648–655 [DOI] [PubMed] [Google Scholar]
- 32.Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis 2003;9:410–419 [PubMed] [Google Scholar]
- 33.Kapphahn RJ, Ethen CM, Peters EA, Higgins L, Ferrington DA. Modified alpha A crystallin in the retina: altered expression and truncation with aging. Biochemistry 2003;42:15310–15325 [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload induced ventricular hypertrophy and congestive heart failure. Circ Res 2007;7:1089–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto C, Yu CR, Shi G, et al. Pertussis toxin is superior to TLR ligands in enhancing pathogenic autoimmunity targeting at a Neo-self antigen by triggering robust expansion of the Th1 cells and their cytokine production. Int J Immunol 2006;177:6896–6903 [DOI] [PubMed] [Google Scholar]
- 36.Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res 2007;26:78–98 [DOI] [PubMed] [Google Scholar]
- 37.Khurana RN, Parikh JG, Saraswathy S, Wu GS, Rao NA. Mitochondrial oxidative DNA damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 2008;49:3299–3304 [DOI] [PubMed] [Google Scholar]
- 38.Ohtaki H, Takeda T, Dohi K, et al. Increased mitochondrial DNA oxidative damage after transient middle cerebral artery occlusion in mice. Neurosci Res 2007;58:349–355 [DOI] [PubMed] [Google Scholar]
- 39.Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2 inhibitable pathway in rat insulin-producing cells. Endocrinology 2000;141:2003–2010 [DOI] [PubMed] [Google Scholar]
- 40.Kurose I, Miura S, Higuchi H. Increased nitric oxide synthase activity as a cause of mitochondrial dysfunction in rat hepatocytes: roles for tumor necrosis factor alpha. Hepatology 1996;24:1185–1192 [DOI] [PubMed] [Google Scholar]