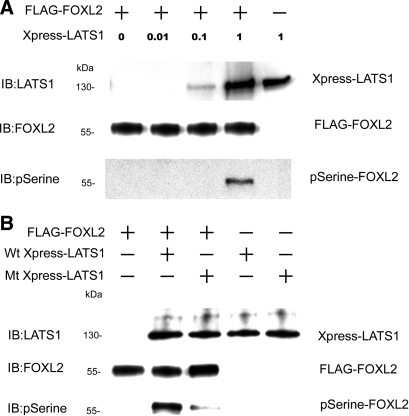
Fig. 3.
FOXL2 is phosphorylated by LATS1. A: CHO cells were transfected with the pcDNA3-His-Xpress LATS1 construct and lysed, and Xpress-tagged LATS1 was purified using a LATS1 antibody for use as the kinase. Dephosphorylated FLAG-tagged FOXL2 proteins were used as substrates in kinase assays. Different amounts of Xpress-tagged LATS1 were mixed with FLAG-tagged FOXL2 for kinase phosphorylation, and the reaction products were analyzed by IB with antibodies to FOXL2, LATS1, and phosphoserine. In the absence of Xpress-LATS1, FOXL2 was not phosphorylated at a serine residue. In the presence of increasing concentrations of Xpress-LATS1, the amount of phosphoserine-FOXL2 (pSerine-FOXL2) increased. B: to determine whether LATS1 is a kinase for FOXL2, CHO cells were transfected with pcDNA3-His-Xpress LATS1 constructs, either the wild-type (Wt Xpress-LATS1) or the kinase-inactive D846A mutant (Mt Xpress-LATS1), lysed, and purified using the Xpress antibody for use as kinases. Xpress-tagged wild-type LATS1 or Xpress-tagged mutant LATS1 D846A was mixed with dephosphorylated FLAG-tagged FOXL2 for kinase phosphorylation. A strong phosphoserine-FOXL2 band was observed when wild-type LATS1 was used as the kinase, but only a very faint band was observed when the same concentration of the D846A mutant LATS1 was used.