Abstract
An emerging literature attests to the ability of psychological stress to alter the inflammatory cytokine environment of the body. While the ability of stress to cause cytokine release is well established, the neural pathways involved in this control have yet to be identified. This study tests the hypothesis that IL-6 neurons of the hypothalamo-neurohypophyseal system (HNS), a neural pathway proposed to secrete IL-6 into the circulation, are activated in response to psychological stress. Colocalization studies confirm robust expression of IL-6 in cell bodies and fibers of vasopressin (but not oxytocin) neurons of the paraventricular (PVN) and supraoptic nucleus (SON) of the rat hypothalamus. In response to restraint, there was a greater increase in c-Fos expression in SON IL-6-positive (IL-6+) neurons. In addition, both psychogenic (restraint) or systemic stress (hypoxia) lead to phosphorylated ERK induction only in IL-6+ magnocellular neurons, indicating selective activation of the MAPK signaling pathway in the IL-6 subset of magnocellular neurons. Finally, restraint upregulated IL-6 mRNA expression in both the PVN and SON, which was accompanied by a four-fold increase in circulating IL-6. The data indicate that noninflammatory stressors selectively activate IL-6 magnocellular neurons, upregulate IL-6 gene expression in the PVN and SON, and increase plasma IL-6. In summary, results show that IL-6 neurons of the HNS are a recruited component of the response to psychological stress.
Keywords: cytokine, magnocellular, supraoptic nucleus, paraventricular nucleus, hypothalamo-neurohypophyseal system
stress causes the release of proinflammatory cytokines. The impact of stress on the inflammatory environment is a key mechanism by which stress negatively affects health (18). Inflammation is a contributing factor in a variety of human diseases (10) and is implicated in the progression of metabolic disorders (4), cardiovascular disease (2), depression (27), and autoimmune disease (9), all disorders that are stress sensitive.
The acute-phase response to psychological stress is quite similar to the acute-phase response to infection, even when the perceived stressor poses no immediate threat to homeostasis. Like infection, stress increases proinflammatory cytokines (31), body temperature (22), activation of the hypothalamo-pituitary-adrenocortical (HPA) axis, and production of acute-phase proteins in the liver (13). Although the magnitude of change in individual factors may vary, there is a large overlap between responses to stress and infection. With infection, IL-1β, IL-6, and TNF-α are the primary mediators of the systemic acute-phase response and are derived from immune cells. In contrast, immune cells collected after psychological stress in humans do not show increased in vitro cytokine production (31), despite the fact that there are increases in the circulating concentration of IL-1β and IL-6. In particular, IL-6 production from immune cells collected in vivo then stimulated in vitro does not increase following stress exposure (31) and may even be downregulated (36), suggesting a nonimmune site of production for stress-induced IL-6.
Central injections that block adrenergic or IL-1β signaling attenuate the IL-6 response to psychological stress (14, 30), indicating that there are central brain pathways that control stress-induced IL-6 release. The expression of IL-6 in the magnocellular nuclei, primarily colocalized with vasopressin (12), suggests that IL-6 may function as a secretory product of the hypothalamo-neurohypophyseal system (HNS). Thus, this study focuses on the IL-6 neurons of the HNS, as a cytokine-expressing neural pathway that could be involved in the stress response. The current study was, therefore, designed to test the hypothesis that IL-6 neurons of the HNS are activated in response to psychological stress, and respond to stress by augmenting IL-6 expression, and that the activation of this pathway would be accompanied by an increase in plasma IL-6.
MATERIALS AND METHODS
Animals
Experiments were completed in adult male Sprague-Dawley rats (275–350 g body wt). Animals were housed 2 per cage on a 12:12-h light-dark cycle. Animals were provided ad libitum access to food and water. Wild-type and IL-6 knockout mice (courtesy Angela Drew, University of Cincinnati) were used to test antibody specificity. All experiments were approved by the University of Cincinnati Animal Care and Use Committee.
Stressors
To determine whether IL-6 neurons of the HNS are stress responsive, we designed our experiments to include a psychological stress challenge (restraint), and a systemic stress challenge (hypoxia), since the recruitment of stress-activated pathways can be unique to the stressor modality. For restraint, animals were placed in clear Plexiglas tubes. Hypoxia consisted of placement of animals in a clear Plexiglas chamber with 8% O2 and 92% N2. Separate groups of animals were used for each experiment so that animals were not exposed to more than a single stressor.
Stress induction of c-Fos in IL-6 neurons.
The colocalization of c-Fos in IL-6 neurons was determined following restraint stress. Animals were placed in restrainers for 2 h or kept in their home cage (n = 10 rats per group). After the 2-h bout of restraint, animals were removed from restrainers and placed back in their home cage for 1 h. At 1 h after stressor cessation, animals were given an overdose of pentobarbital sodium; they were perfused with saline and then 3.7% formaldehyde in 50 mM potassium phosphate buffered saline (KPBS) solution. Brains were removed and placed in 3.7% formaldehyde, 50 mM KPBS solution and stored at 4°C overnight. Brains were then placed in 30% sucrose, 50 mM KPBS solution and kept at 4°C. Brains were cut into 25-μm-thick sections (SM2000R, Leica Microsystems), and stored in cryoprotectant at −20°C until analysis. Brain sections were used for dual immunohistochemistry to determine whether stress increases c-Fos immunoreactivity in IL-6 neurons.
Immunohistochemistry
Immunohistochemistry was completed with free-floating sections, and all rinses consisted of 5 × 5 min in 50 mM KPBS. Sections were removed from cryoprotectant and rinsed. For antigen retrieval, sections were incubated in 50 mM Na Citrate, pH 9.0, for 10 min at 80°C, then allowed to cool at room temperature for 20 min (16). Sections were rinsed, then placed in blocking solution (0.5% BSA, 0.2% Triton-X 100, 50 mM KPBS) for 1 h. Sections were incubated overnight in blocking solution at 4°C with IL-6 (1:250) and c-Fos (1:10,000) antibodies. The IL-6 antibody was an affinity-purified goat polyclonal antibody directed against the C-terminus of rat IL-6 (IL-6, R-19: sc-1266; Santa Cruz Biotechnology), and the c-Fos antibody was a rabbit polyclonal antibody (Santa Cruz). After the overnight incubation, sections were rinsed, then incubated with secondary antibodies (Cy3 anti-goat, 1:1,000; Cy5 anti-rabbit, 1:600; Jackson ImmunoResearch). After rinsing, sections were mounted on slides and allowed to dry; then gelvatol and coverslips were added. The specificity of the IL-6 antibody was tested in control tissue from wild-type and IL-6 knockout mice.
For triple labeling, an additional overnight incubation step was added. The first overnight incubation was with goat anti-IL-6 (1:250) and rabbit anti-oxytocin (1:10,000; ab11143; Abcam) antibodies, followed by incubation with secondary antibodies (Cy3 anti-goat, 1:1,000; Cy5 anti-rabbit, 1:600; Jackson ImmunoResearch). After washing, sections were placed in blocking solution and then incubated overnight with a rabbit anti-vasopressin neurophysin antibody (1:25,000, courtesy Alan Robinson) followed by incubation with a secondary antibody (1:500; anti-rabbit Alexa 488; Invitrogen).
Image Capture and Analysis
Images of the paraventricular nucleus (PVN; −1.8 mm from bregma) and supraoptic nucleus (SON; −1.3 mm from bregma) were collected with a fluorescent microscope (Axio Imager Z1, Zeiss) based on rat brain atlas (26). Black and white images were collected, and false color was added. Cell counts were obtained for 1) IL-6-positive neurons; 2) IL-6-positive neurons with c-Fos immunoreactivity; 3) c-Fos-positive neurons without IL-6 immunoreactivity. For each animal, bilateral cell counts from one section were obtained from the PVN and SON, and counts from each side combined for analysis. In addition to cell counts, the area of the paraventricular or supraoptic nucleus used for counting was also obtained, so that counts could be expressed per unit area. Image capture and counting were completed by a researcher blinded to the animal's group identification.
Stress activation of MAPK signaling pathway.
To assess the activation of the MAPK cell signaling pathway in IL-6 neurons during stress, brains were collected to examine the stress-induced phosphorylation of ERK in IL-6 neurons. In this experiment, animals were exposed to restraint or hypoxia for 0 min, 15 min, or 30 min (n = 6 rats per group per time point). In addition, a recovery set of animals was also included. Recovery animals were exposed to restraint or hypoxia for 30 min and then allowed to recover for 90 min. At the corresponding time points, animals were given an overdose of pentobarbital sodium and then perfused, as described above. The phosphorylation of ERK occurs on a faster timescale than the de novo synthesis of c-Fos protein; therefore, different timeframes were used for the c-Fos and MAPK studies. Brains were sectioned and used for dual immunohistochemistry for pERK and IL-6. Immunohistochemistry, image capture and analysis were completed as described above with the replacement of the c-Fos antibody with a pERK antibody. The pERK antibody was a monoclonal mouse antibody (1:1,500, p42/44 MAPK, Cell Signaling) and was detected with a Cy5 anti-mouse secondary antibody (Jackson Immunoresearch).
IL-6 mRNA expression in the hypothalamus.
Rats were placed in restrainers for 2 h or remained in their home cage (n = 8 restraint; 11 control). At the 2-h time point, animals were rapidly decapitated, trunk blood was collected, and brains were immediately removed. Trunk blood was collected in 15-ml falcon tubes containing 150 μl of 100 mM EDTA solution. Approximately 4–6 ml of blood was collected per animal. Blood was centrifuged at 1,850 g for 20 min at 4°C, and plasma aliquots were frozen. Brains were rinsed in cold saline, and a coronal section of the hypothalamus was obtained (from approximately −1.0 to −2.0 mm from bregma) based on the rat brain atlas (26). SON-enriched and PVN-enriched dissections were excised from the coronal section and placed in cold RNAlater (Ambion) and stored at −20°C. Plasma aliquots were used to measure the concentration of IL-6, and brain dissections were used for quantifying IL-6 mRNA in the PVN and SON, as described below.
Quantitative RT-PCR
Brain sections were cut (∼1 mm thick), and PVN and SON were dissected using anatomical landmarks based upon the rat brain atlas (26). Tissue dissections were homogenized, and total RNA was isolated (RNeasy Mini Kit; Qiagen) and then cDNA synthesized (iScript cDNA synthesis kit; Bio-Rad), both according to the manufacturer's instructions. The concentration and quality of RNA and cDNA samples were measured by spectrophotometry (ND-1000 UV-Vis spectrophotometer, NanoDrop Technologies). Minus RT reactions were included to verify removal of DNA contamination. Quantitative RT-PCR analysis was completed with the iCycler iQTM multicolor real-time PCR detection system (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). Primers were intron spanning and designed using Primer3 software (28) and ordered from Sigma-Aldrich. Oligonucleotide sequences were IL-6, forward: 5′GCGATGATGCACTGTCAGA3′ from exon 3, and reverse: 3′ACTCCAGAAGACCAGAGCAGAT3′ from exon 4; and L32, forward: 5′CATCGTAGAAAGAGCAGCAC3′ and reverse: 5′GCACACAAGCCATCTATTCAT3′.
The Ct value analysis, PCR efficiency, linearity, and slopes of the standard curve were calculated using Bio-Rad iCycler software built in iCycler iQ Multi-Color real-time PCR Detection System. Assay sensitivity was determined by the serial dilution of pooled RNA from PVN and SON dissections obtained from spare rats. Standard curve analysis showed a linear relationship between the threshold cycles and the serial dilution of the initial cDNA. For quantitative RT-PCR analysis, L-32, a house-keeping gene, was applied as reference gene to normalize the data. RNA levels were first measured by the reference gene, L-32, and no differences were detected between groups (average Ct value for L32 was 18). Threshold cycle readings for each of the unknown samples were used (average Ct value for IL-6 was 29), and results were transferred to and calculated in Excel using the ΔΔCt method (23). Values shown in results were calculated using L-32 as an internal standard and expressed as percent of control.
ELISA
The concentration of IL-6 in plasma was measured by enzyme-linked immunosorbent assay (ELISA) that was run according to manufacturer's instructions (Quantikine Rat IL-6; R&D Systems). A MRX revelation plate reader (Dynex Technologies) was used to measure optical density (OD), and a standard curve was generated (15.6–1,000 pg/ml) to calculate results.
Statistical Analysis
Data are presented as means ± SE. Plasma IL-6, mRNA, and c-Fos data were analyzed by independent sample t-test. If data failed the normality or equal variance test, the Mann-Whitney U test was used for analysis (plasma IL-6). The pERK data were square root transformed to normalize the variance and subsequently analyzed by three-way ANOVA (stressor, time, nucleus) for both IL-6-positive (IL-6+) and IL-6 negative (IL-6−) neurons. Statistical analysis was completed using SigmaStat 9.0 software, and the level of significance set at P ≤ 0.05.
RESULTS
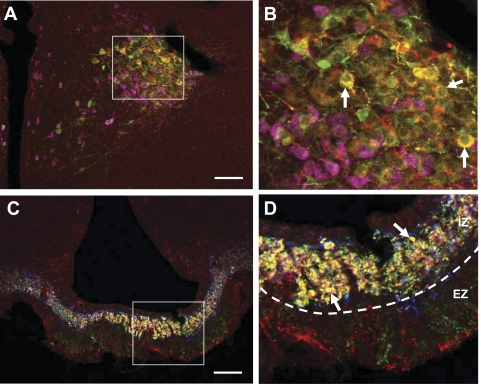
Initial studies assessed expression of IL-6 immunoreactivity in specific HNS cell populations. Hypothalamic sections were triple labeled for IL-6, oxytocin, and vasopressin. Representative images show that the majority of IL-6 neurons show vasopressin immunoreactivity, whereas IL-6 and oxytocin were not colocalized to any significant degree (Figs. 1, A and B, Fig. 2). We also observed a small population of IL-6+ magnocellular neurons that were not immunoreactive for vasopressin or oxytocin (Fig. 1B). Consistent with the IL-6 vasopressin colocalization in PVN and SON cell bodies, IL-6 and vasopressin were also colocalized in the internal (but not external) zones of the median eminence, consistent with a common origin in magnocellular (but not parvocellular) vasopressin neurons (Fig. 1, C and D). Again, IL-6 oxytocin colocalization was not evident (Fig. 1D). There were immunoreactive neurons in the parvocellular division of the PVN (Fig. 1). However, cell morphology suggests that these are magnocellular neurons and that IL-6 does not colocalize with corticotropin-releasing hormone (CRH) in parvocellular neurons, consistent with previous results (12).
Fig. 1.
IL-6 expression in the hypothalamo-neurohypophyseal system. A: immunoreactivity for IL-6 (red), vasopressin (green), and oxytocin (pink) in the magnocellular division of the paraventricular nucleus. Yellow cells indicate colocalization of IL-6 and vasopressin, and examples are noted by white arrows (B). Red neurons indicate IL-6-positive (IL-6+) neurons that lack immunoreactivity for vasopressin and oxytocin (B). C: immunoreactivity for IL-6 (red), vasopressin (green), and oxytocin (blue) in the median eminence. Yellow shows colocalization of IL-6 and vasopressin in the internal zone of the median eminence, as noted by white arrows (D). Oxytocin immunoreactivity did not colocalize with IL-6 or vasopressin (D). Vasopressin immunoreactivity in the external zone did not colocalize with IL-6 (D). Images on right (B and D) are magnified views from the white boxed area. IZ, internal zone; EZ, external zone. Scale bars: 200 μm (A); 100 μm (C).
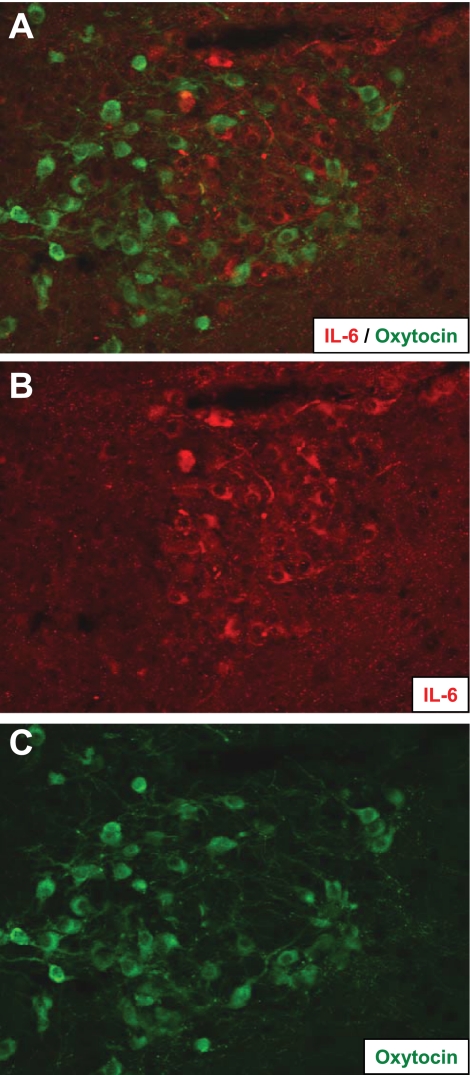
Fig. 2.
A: IL-6 and oxytocin do not colocalize. B: IL-6 immunoreactivity (red) was observed in subset of neurons that did not express oxytocin (C: green).
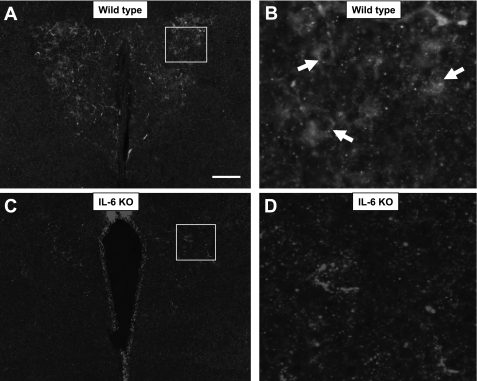
We tested the IL-6 antibody in wild-type and IL-6 knockout (KO) mice to confirm antibody specificity (Fig. 3). Results show IL-6 immunoreactivity in neurons of the PVN of wild-type mice (Fig. 3, A and B). Importantly, neural IL-6 immunoreactivity is not observed in IL-6 knockout mice (Fig. 3, C and D), verifying that the antibody was specific for IL-6.
Fig. 3.
Specificity of the IL-6 antibody. IL-6 immunoreactivity (white) in magnocellular neurons of the paraventricular nucleus (PVN) is observed in wild-type mice (A, B) but is eliminated in IL-6 knockout mice (C, D). Scale bar: 200 μm.
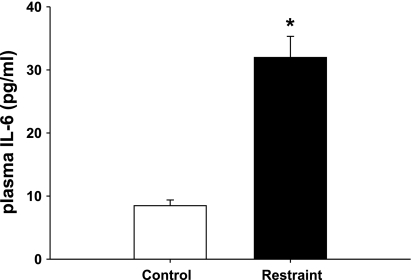
We tested the ability of acute restraint to directly elicit an IL-6 response. Our data indicate an almost four-fold increase in plasma IL-6 levels in response to 2 h of restraint [T (17) = 124.0, P < 0.001; Fig. 4], consistent with centrally mediated, psychogenic stress-induced cytokine release.
Fig. 4.
Effect of acute stress on plasma IL-6. In response to 2 h of restraint, plasma IL-6 was significantly increased compared with control animals that remained in their home cage (*P < 0.001; n = 10).
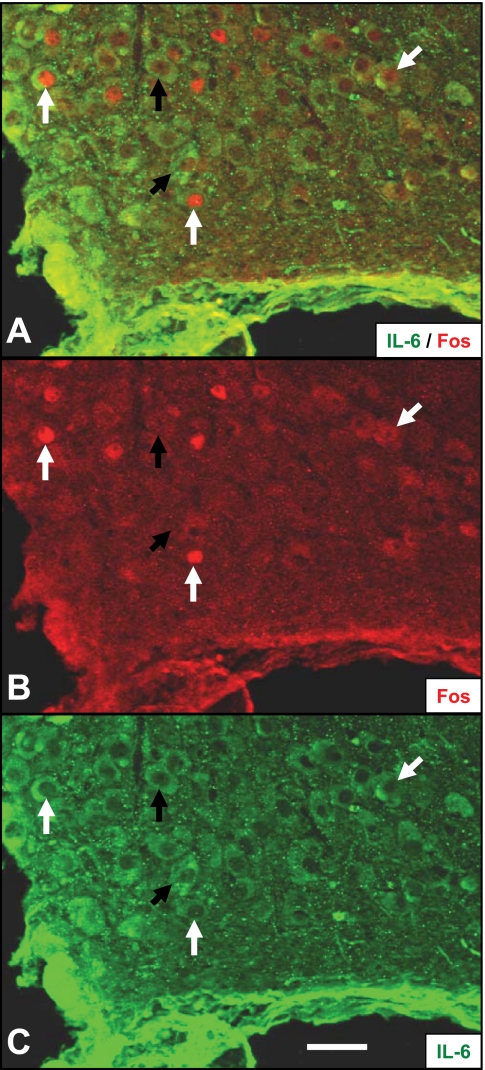
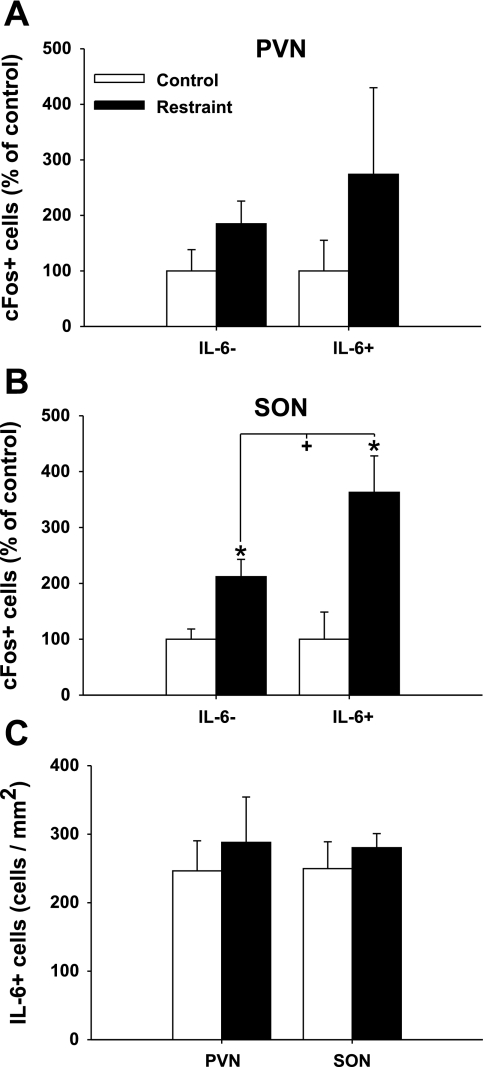
To determine whether IL-6 neurons in the hypothalamus are stress responsive, we examined the expression of c-Fos, a marker of neuronal activation (6), in IL-6+ and IL-6− neurons following restraint stress (Fig. 5). Counts of the number of IL-6 and c-Fos-immunoreactive cells indicate a significant increase in number of c-Fos expressing IL-6− [t (17) = 3.029, P < 0.01] and IL-6+ [t (17) = 3.174, P < 0.01] neurons in the SON (Fig. 6). The increase in c-Fos immunoreactivity in IL-6− and IL-6+ neurons was twofold and 3.5-fold, respectively, suggesting selective recruitment of IL-6 neurons, with the difference in c-Fos induction between the two cell types reaching statistical significance [t (18) = 2.090, P = 0.05]. In the PVN, there were no statistically significant changes in the number of c-Fos-expressing IL-6- or IL-6+ neurons, although the pattern resembled the changes observed in the SON (Fig. 6). Restraint stress did not increase or decrease the total number of detectable IL-6-immunoreactive neurons in the PVN or SON (Fig. 6).
Fig. 5.
Stress activation of IL-6 neurons in the supraoptic nucleus. Immunoreactivity for c-Fos (B), IL-6 (C) and merged image (A) showing colocalization of nuclear c-Fos signal in IL-6+ neurons following restraint. White arrows denote examples of IL-6+ neurons that are positive for c-Fos; black arrows denote Fos negative IL-6+ neurons. Scale bar: 100 μm.
Fig. 6.
Differential c-Fos induction in IL-6-positive magnocellular neurons following acute stress. Comparison of the number of IL-6- and IL-6+ neurons that colocalize c-Fos in the PVN (A) and SON (B). In the SON, restraint increases the number of c-Fos-positive neurons. Notably, a significantly greater proportion of IL-6+ neurons were activated by stress. Data are presented as a percentage of respective control group. C: total number of IL-6 neurons/mm2 in the PVN and SON in control and restraint-stressed animals. *P < 0.01 vs. control within cell type; +P = 0.05 vs. cell type within restraint (n = 10).
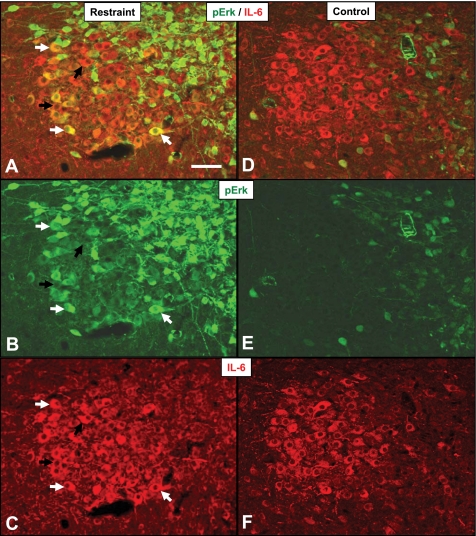
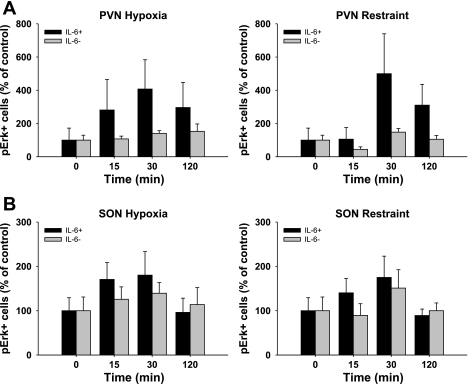
The expression of IL-6 is known to be regulated by the MAPK signaling pathway (34), at least in epithelial cells in vitro. Therefore, we use a histochemical index of ERK phosphorylation to determine whether stress activates the MAPK pathway in IL-6+ neurons (Fig. 7). Regardless of stressor modality [psychogenic (restraint) vs. systemic (hypoxia)], there was a significant main effect of time [F (3, 92) = 3.250, P < 0.05] on pERK localization in IL-6+ neurons, consistent with stress activation of the MAPK pathway in IL-6+ neurons (Fig. 8). Importantly, the induction of pERK did not occur in magnocellular neurons lacking IL-6 immunoreactivity, again suggesting specialization of IL-6-expressing HNS neurons. There was no effect (P > 0.05) of stressor type or hypothalamic region on pERK localization in IL-6+ or IL-6- magnocellular neurons. Figure 7 also shows an increase in pERK induction in the smaller parvocellular neurons of the PVN (Fig. 8, A and B).
Fig. 7.
Activation of MAPK signaling pathway in IL-6 neurons in stress (A, B, C) animals and controls (D, E, F). Immunoreactivity for phosphorylated ERK (B, E; green) and IL-6 (C, F; red) in the PVN. Merged images show pERK induction in response to stress (A) compared with control animals (D). White arrows denote examples of IL-6+ neurons colocalized with pERK; black arrows denote pERK negative IL-6+ neurons. Scale bar: 100 μm.
Fig. 8.
Effect of acute stress on ERK phosphorylation in IL-6- and IL-6+ neurons. Data are presented as a percentage of control (0 min) for the response to hypoxia (left) and restraint (right) in the PVN (A) and SON (B). There was a significant main effect of time on pERK induction in IL-6+ neurons that did not occur in IL-6- neurons (n = 6).
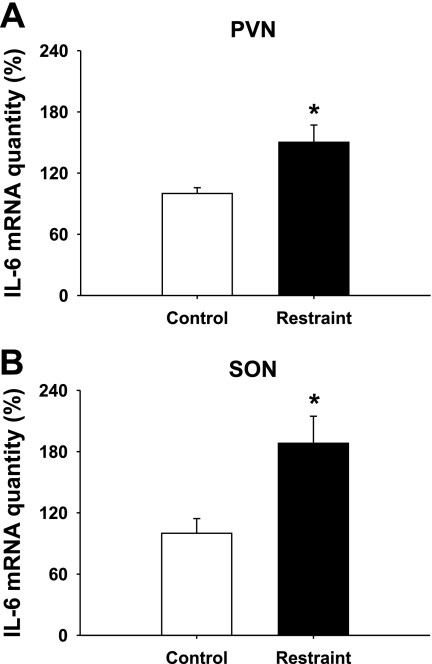
We went on to test the effect of stress on IL-6 mRNA expression in hypothalamic microdissections, including the PVN and SON. Similar to the increase in activation of IL-6 neurons in the hypothalamus, IL-6 mRNA in both PVN [t (17) = −3.181, P < 0.01] and SON [t (17) = −3.127, P < 0.01] regions is upregulated in response to restraint (Fig. 9).
Fig. 9.
Acute stress and IL-6 mRNA expression in the hypothalamus. Restraint stress increased IL-6 mRNA in both the PVN (A; *P < 0.01) and SON (B; *P < 0.01). Data are presented as means ± SE (n ≥ 8).
DISCUSSION
The current study demonstrates that interleukin-6 neurons of the HNS are sensitive to psychogenic stress. Activation of IL-6 neurons was demonstrated by an increase in c-Fos, a marker of neuronal activation (6), as well as by the phosphorylation of ERK, a critical component of the MAPK cell signaling pathway (34). Furthermore, IL-6 mRNA expression in the hypothalamus is upregulated in response to stress, and activation of IL-6 magnocellular neurons in the hypothalamus is accompanied by an increase in plasma IL-6. Thus, IL-6 neurons of the HNS are involved in the response to psychological stress.
Expression of IL-6 in the HNS is documented in rats (11, 12), as well as in pigs (15). Our results from IL-6 knockout mice confirm that the antibody employed detects authentic IL-6, confirming cytokine expression in HNS neurons. Consistent with previous results, IL-6 is predominantly colocalized with vasopressin in magnocellular neurons and their fibers in the internal zone of the median eminence. We did not observe coexpression of IL-6 and oxytocin. Interestingly, there was a subset of IL-6 neurons that did not demonstrate immunoreactivity for vasopressin or oxytocin, suggesting the existence of a separate, albeit small, population of IL-6-producing neurons. The neurotransmitter phenotype of these IL-6-positive, vasopressin- and oxytocin-negative neurons is currently unknown. We should note that these neurons are unlikely to coexpress CRH, as IL-6 immunoreactivity was observed in magnocellular, not parvocellular, neurons, and previous studies did not demonstrate CRH-IL-6 colocalization in the PVN (12).
Our overarching hypothesis is that central neural pathways control stress-induced cytokine production. In this study, we provide evidence that the IL-6 magnocellular neurons are stress responsive. Like the neuropeptides vasopressin and oxytocin, IL-6 is produced in magnocellular neurons and transported to the neurohypophysis, where it is in position to be released into the general circulation (11, 12, 15). Generally, magnocellular neurons of the PVN and SON release neuropeptides in the neurohypophysis, where they enter the general circulation and act on their peripheral targets (19). The expression pattern of IL-6 in the HNS suggests that IL-6 may be a secreted product from the neural lobe. It is also possible that IL-6 functions within the PVN and SON to regulate the expression of hypothalamic peptides (1). The fact that IL-6 is extensively colocalized with AVP in cell bodies and is colocalized with AVP in fibers and terminal boutons suggests that IL-6 may be coreleased with AVP (12). Thus, the neuroanatomical data suggest that IL-6 is released from the neurohypophysis to act on peripheral targets.
The hypothesis that IL-6 is a secretory product of the HNS was originally suggested by Ghorbel et al. (11). In their study, IL-6 was one of nine candidate genes in the hypothalamus that was identified as being regulated by dehydration. Authors went on to show that IL-6 expression in the hypothalamus, median eminence, and posterior pituitary was regulated by 3 days of fluid deprivation, consistent with regulation by a nonimmune systemic stressor (i.e., dehydration). We previously demonstrated that IL-6 content in the median eminence decreased after a single bout of exercise stress in pig (15), consistent with rapid engagement of HNS IL-6 by acute stress. The current study goes on to show that IL-6 neurons of the HNS are, indeed, recruited in response to a single psychogenic stressor, consistent with engagement of cytokine release by purely neuronal mechanisms, putatively initiated at the level of the forebrain (32). The demonstration that IL-6 neurons of the HNS are stress responsive does not exclude the possibility that plasma IL-6 could be derived from other tissue sources (e.g., splenic lymphocytes).
Stress activation of HNS neurons is limited relative to other stress-activated hypothalamic neurons (e.g., parvocellular PVN corticotrophin-releasing hormone neurons). In general, a small proportion of AVP and OT neurons are activated by stress (6). In this study, we show a stress-induced increase in c-Fos and pERK in IL-6 neurons of the HNS. Of interest, stress activation of IL-6 neurons occurred to a greater extent than the general population of magnocellular neurons, demonstrating selective engagement of IL-6 cells by acute stress exposure. The increased selective recruitment suggests a greater role of the IL-6 subset of neurons in the acute stress response compared with the general population of magnocellular neurons in the HNS. Further, stress activation of IL-6 neurons occurred in response to both restraint and hypoxia, suggesting that the HNS IL-6 neurons are involved in the response to both psychological and systemic stressors.
Increased stress-selective recruitment of IL-6 neurons may be the result of a unique innervation pattern and/or selective expression of receptors in IL-6+ neurons. Candidate ligands for stress activation of IL-6 neurons include noradrenaline and IL-1β, molecules capable of activating HNS neurons (3, 21) and known to induce c-Fos and activate the MAPK pathway (17, 34). Blockade of either ligand through intracerebroventricular injections of a β-adrenergic blocker or IL-1 receptor antagonist attenuates the plasma IL-6 response to psychological stress (14, 30), providing strong evidence for endogenous roles of noradrenaline and IL-1β in the central regulation of stress-induced IL-6. Furthermore, IL-1β, a known inducer of IL-6, increases in the hypothalamus in response to acute stress, raising the possibility that hypothalamic IL-1β controls IL-6 release from magnocellular neurons (24, 25). It is not yet known whether IL-6-positive magnocellular neurons are selectively responsive to noradrenaline, or exhibit preferential noradrenergic innervation or adrenergic receptor expression.
The functional role of IL-6 production in response to stress is still in the process of being defined. During stress exposure, IL-6 has been proposed to function in the regulation of the hepatic acute phase response (13), metabolism (33), and blood pressure regulation (20). Because the stress-induced increase in IL-6 occurs at a slower rate than sympathoadrenal and corticosteroid responses, it is possible that IL-6 only begins to have a functional role during stressors of extended duration or high intensity. Alternatively, it is possible that the critical role for IL-6 may begin upon stressor cessation, where IL-6 may modify the tissue effects of corticosteroids (29) and/or help the individual adapt and recuperate from stress exposure (7). Future studies are needed to continue to define the functional role of stress-induced IL-6.
The effect of stress on inflammatory regulators is a mechanism by which stress can directly impair health (8). In general, alterations in inflammatory cytokine production are observed across a wide spectrum of diseases. Human studies note that changes in the inflammatory cytokine environment in response to chronic stress have detrimental effects on health and well being (18). Overproduction of IL-6, a key inflammatory cytokine, is implicated as a mechanism by which stress contributes to disease progression in heart disease (35), depression (27), and cancer (5). Thus, identification of the stress pathways that influence the inflammatory environment would have great impact potential for human health.
Perspectives and Significance
In summary, our data indicate that acute psychogenic stress activates IL-6 magnocellular neurons in the hypothalamus and increases plasma IL-6. Stress activation led to selective recruitment of neurons that express IL-6, and the data show that IL-6 mRNA expression in the paraventricular and supraoptic regions is stress regulated. Thus, our current study demonstrates that IL-6 neurons of the HNS are a recruited component of the stress response and are uniquely positioned to provide a neural link between stress and inflammatory responses.
GRANTS
This work was supported by the National Institute of Mental Health National Institutes of Health (NIH) Grants MH069725 and MH084515 and the National Institute of Diabetes and Digestive and Kidney Diseases NIH Grant DK058903.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Anne Christiansen Annette DeKloet, Kenny Jones, Eric Krause, Ben Packard, and Yve Ulrich-Lai for the assistance with animal experiments.
REFERENCES
- 1.Benrick A, Schele E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, Jansson JO. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol 21: 620–628, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res 52: 1–23, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Chakfe Y, Zhang Z, Bourque CW. IL-1β directly excites isolated rat supraoptic neurons via upregulation of the osmosensory cation current. Am J Physiol Regul Integr Comp Physiol 290: R1183–R1190, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chan JC, Cheung JC, Stehouwer CD, Emeis JJ, Tong PC, Ko GT, Yudkin JS. The central roles of obesity-associated dyslipidaemia, endothelial activation and cytokines in the metabolic syndrome—an analysis by structural equation modelling. Int J Obes Relat Metab Disord 26: 994–1008, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer 104: 305–313, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Deak T. From classic aspects of the stress response to neuroinflammation and sickness: implications for individuals and offspring. Int J Comp Psychol 20: 96–110, 2007 [Google Scholar]
- 8.Dhabhar FS. A hassle a day may keep the pathogens away: The fight-or-flight stress response and the augmentation of immune function. Integr Comp Biol 49: 215–236, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann NY Acad Sci 966: 290–303, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 12: 255–269, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, Murphy D. Microarray analysis reveals interleukin-6 as a novel secretory product of the hypothalamo-neurohypophyseal system. J Biol Chem 278: 19280–19285, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Hernandez T, Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, Abreu P, del Mar Perez-Delgado M, Rancel-Torres N, del Carmen Gonzalez M. Interleukin-6 and nitric oxide synthase expression in the vasopressin and corticotrophin-releasing factor systems of the rat hypothalamus. J Histochem Cytochem 54: 427–441, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez J, Carrasco J, Belloso E, Giralt M, Bluethmann H, Kee Lee D, Andrews GK, Hidalgo J. Metallothionein induction by restraint stress: role of glucocorticoids and IL-6. Cytokine 12: 791–796, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa I, Kitamura H, Kimura K, Saito M. Brain interleukin-1 is involved in blood interleukin-6 response to immobilization stress in rats. Jpn J Vet Res 49: 19–25, 2001 [PubMed] [Google Scholar]
- 15.Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH. Sex difference in link between interleukin-6 and stress. Endocrinology 148: 3758–3764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93: 149–162, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Khorchid A, Larocca JN, Almazan G. Characterization of the signal transduction pathways mediating noradrenaline-stimulated MAPK activation and c-fos expression in oligodendrocyte progenitors. J Neurosci Res 58: 765–778, 1999 [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA 100: 9090–9095, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs KJ. Neurohypophyseal hormones in the integration of physiological responses to immune challenges. Prog Brain Res 139: 127–146, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lee DL, Leite R, Fleming C, Pollock JS, Webb RC, Brands MW. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension 44: 259–263, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Leibowitz SF, Eidelman D, Suh JS, Diaz S, Sladek CD. Mapping study of noradrenergic stimulation of vasopressin release. Exp Neurol 110: 298–305, 1990 [DOI] [PubMed] [Google Scholar]
- 22.LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav 47: 957–961, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci 18: 2239–2246, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991: 123–132, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 2005 [Google Scholar]
- 27.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Smith C, Wilson NW, Louw A, Myburgh KH. Illuminating the interrelated immune and endocrine adaptations after multiple exposures to short immobilization stress by in vivo blocking of IL-6. Am J Physiol Regul Integr Comp Physiol 292: R1439–R1447, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Soszynski D, Kozak W, Rudolph K, Conn CA, Kluger MJ. Open field-induced rise in body temperature and plasma IL-6 is mediated by beta-adrenoceptors in the brain. Ann NY Acad Sci 813: 413–419, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 21: 901–912, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88: 3005–3010, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Yang HT, Cohen P, Rousseau S. IL-1β-stimulated activation of ERK1/2 and p38α MAPK mediates the transcriptional up-regulation of IL-6, IL-8 and GRO-α in HeLa cells. Cell Signal 20: 375–380, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148: 209–214, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 133: 2523–2530, 1993 [DOI] [PubMed] [Google Scholar]