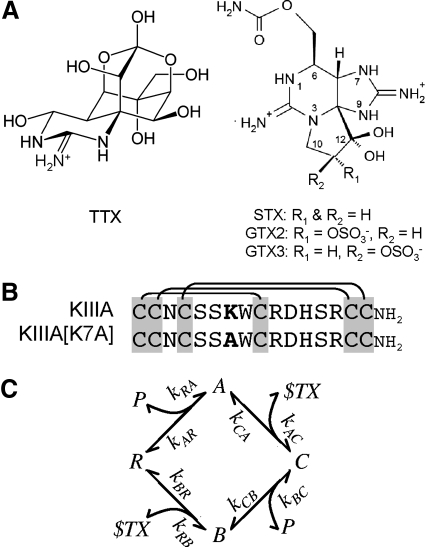
Fig. 1.
Structures of guanidinium alkaloids and peptides whose activities were examined in this study. A: tetrodotoxin (TTX, left) and saxitoxin (STX) and GTX2/3 (STX congener with a net +1 charge; right). At neutral pH, TTX has a single positive charge, whereas STX has 2. GTX2/3 is a mixture of epimers GTX2 and GTX3 and differs from STX by having a sulfate group, so its net charge is +1 (Hall et al. 1990). B: aligned sequences of μ-conotoxin KIIIA and its point mutant KIIIA[K7A]. These peptides are identical except for bold residue, which is Lys7 in KIIIA and Ala7 in KIIIA[K7A]. Three disulfide bridges characteristic of all μ-conotoxins are indicated by lines linking Cys residues. Carboxyl terminals of both peptides are amidated. C: proposed reaction scheme for the syntoxin interactions between alkaloid and peptide when they bind to NaV1.2. The channel (i.e., receptor) is represented by R and its ligands by P for μ-conopeptide and $TX for alkaloid (TTX or STX). A is the binary peptide·channel complex, B is the binary alkaloid·channel complex, and C is the ternary peptide·alkaloid·channel complex. The formation of the same ternary complex, independent of order of ligand addition, was inferred from the interactions of TTX and KIIIA or KIIIA[K7A] with NaV1.2 (Zhang et al. 2009) and is also consistent with the results in the present experiments with STX and GTX2/3. The notation for the rate constants correspond to that in the formulae, used to numerically simulate the kinetics of the reactions, in methods.