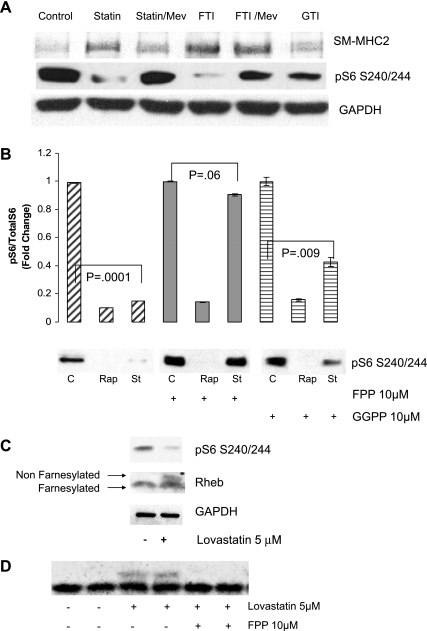
Fig. 4.
Role for farnesylation in lovastatin-induced VSMC differentiation and mTORC1 inhibition. A: human iliac VSMC were cultured in the presence of vehicle (ethanol), 5 μM lovastatin, 10 μM FTI277 (FTI), or 10 μM GTI 298 (GTI) for 8 h. Where indicated, VSMC were pretreated for 1 h with 10 μM mevalonate (Mev) before the other drug treatment. Equal amounts of protein (as determined by Bradford analysis) were loaded for each lane and analyzed by immunoblotting using antibodies against SM2-MHC and pS6 (S240/244). GAPDH served as a loading control. B: human VSMC were pretreated with 10 μM farnesylpyrophosphate (FPP) or 10 μM geranylgeranyl pyrophosphate (GGPP) for 1 h. Vehicle (ethanol; C) or 5 μM lovastatin (St) was then added for an additional 8 h and analyzed by immunoblotting as above using pS6 (S240/244) antibody. GAPDH served as a loading control. A representative blot is shown. The graph shows quantitation of Western blots from 3 experiments. C: VSMC were treated with 5 μM lovastatin or vehicle for 24 h as indicated. Lysates were analyzed by immunoblotting as above using antibodies to Rheb, pS6, and GAPDH. Top arrow indicates nonfarnesylated Rheb and bottom arrow indicates farnesylated Rheb (Rheb cleavage precedes farnesylation). D: human VSMC were pretreated with 10 μM FPP. Vehicle (ethanol) or 5 μM lovastatin was then added for an additional 8 h. Lysates were analyzed by immunoblotting as above using anti-Rheb antibody.