Table 1.
Alkoxy- and acyloxyarylation with various nucleophiles.a
 | |||
|---|---|---|---|
| entry | R | product | yield (%) |
| 1 | Me | 7 | 79 |
| 2 | Et | 8 | 85 |
| 3 | iPr | 9 | 90 |
| 4 | (Me)3CCH2 | 10 | 91c |
| 5 | tBu | 11 | 33b |
| 6 |
|
12 | 85 |
| 7 |
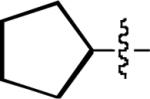
|
13 | 85 |
| 8 |

|
14 | 88 1:1 d.r. |
| 9 | Me(CO)- | 15 | 62 |
| 10 | Et(CO)- | 16 | 69 |
| 11 | iPr(CO)- | 17 | 50 |
| 12 | Ph(CO)- | 18 | 48c |
100 μmol of alkene, 0.1M in 9:1 MeCN:ROH at 50°C for 14h; 2 equiv PhB(OH)2, 2 equiv. Selectfluor®. The catalyst and PhB(OH)2 were added in two portions at t = 0h and 2h.
Yield determined by 1H-NMR versus an internal standard (nitrobenzene).
10 equiv. ROH used.
