Table 3.
Alkoxy- and acyloxyarylation of simple alkenes.a
|
| ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | product | yield (%) | ||
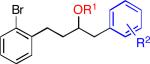
| 1 |
|
|
4-Br | 25 | 69 | |
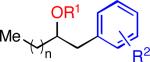
| 2 | Me | 2-CO2Me | 26 | 78 | ||
| 3 | Me(CO) | 4-Br | 27 | 51b | ||
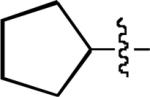
| 4 |
|
n = 5 |

|
4-Br | 28 | 76 |
| 5 | n = 5 | (Me)3CCH2 | 4-Br | 29 | 73 | |
| 6 | n = 9 | (Me)3CCH2 | H | 30 | 66 | |
| 7 | n = 5 | Me(CO) | 4-Br | 31 | 53b | |
100 μmol of alkene, 0.1 M in 9:1 MeCN:R1OH at 50 °C for 14h; 2 equiv. ArB(OH2), 2 equiv. Selectfluor®. The catalyst and ArB(OH)2 were added in 2 portions at t = 0 and 2h.
Only 5 equiv R1OH used, 0.1 M in MeCN.
