Table 4.
Scope of hydroxyarylation reaction.a
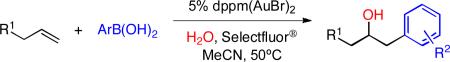 | |||
|---|---|---|---|
| entry | product | yield (%) | |
| 1 |
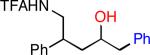
|
32 | 88b |
| 2 |

|
33 | 85c |
| 3 |
|
34 | 67d |
| 4 |
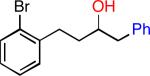
|
35 | 76 |
| 5 |
|
36 | 73 |
| 6 |
|
37 | 72 |
| 7 |
|
R = 3-F, 38 | 83 |
| 8 | R = 4-Me, 39 | 71 | |
At 0.1 M alkene in MeCN, 10 equiv. H2O, catalyst (2.5 mol%/addition) and ArB(OH)2 (1 equiv./addition) were added in 2 portions each, at t = 0h and 1h.
Isolated as a 1.2:1 mixture of diastereomers.
3 portions of catalyst (2.5 mol%/addition) and ArB(OH)2 (1 equiv./addition).
Using (4-CF3C6H4)3PAuBr as catalyst, 2 portions of 5 mol% each.
