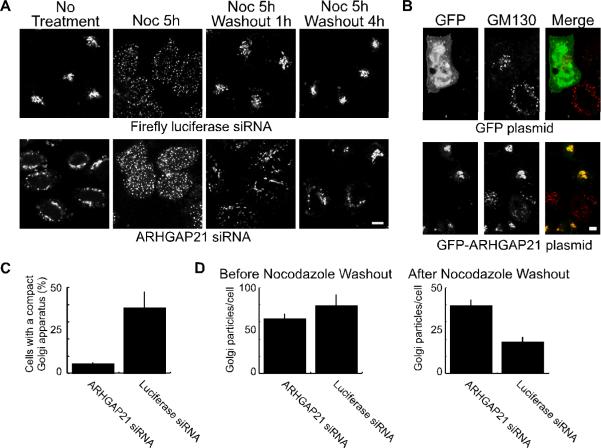
Figure 1. Dynein-dependent Golgi positioning involves ARHGAP21.
(A) Hela cells were transfected with siRNA against ARHGAP21 or a control siRNA against luciferase as indicated. The cells were treated with nocodazole (20 μM) for 5 hours to disperse the Golgi apparatus, and the nocodazole was washed away for the indicated time. At each time point, cells were fixed and Golgi membranes were labeled with GM130 antibody. Shown are confocal micrographs. (B) Hela cells were transfected with siRNA as in (A). After 24 hours the cells were transferred to new plates and transfected with plasmids encoding GFP-ARFBD/GAP domain of ARHGAP21 or GFP alone as indicated. The cells were fixed after 24 additional hours and decorated with antibodies against the Golgi marker, GM130. The size bar equals 5 μm. (C) The fraction of cells containing a compact Golgi apparatus at 1 hour after nocodazole washout was determined for ARHGAP21 and luciferase siRNA expressing HelA cells. The GM130-labeled Golgi complex was defined as compact if it could fit inside a circle with a diameter of 10 μm. Shown is the average from 3 experiments; the bars indicate standard error. (D) The number of Golgi particles per cell was determined using a particle counter plugin for ImageJ. Shown is the average from 3 experiments of 10 cells each; the bars indicate standard error. There was no significant change in the average number of particles/cell before nocodazole washout (p=0.3245). After the one hour nocodazole washout, cells treated with ARHGAP21 siRNA had significantly more particles than cells treated with luciferase siRNAs (p<0.006).