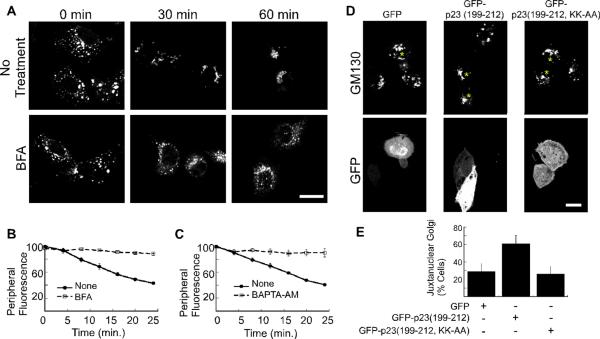
Figure 3. Golgi positioning depends on ARF1 activity and coatomer.
(A) NRK cells were treated with nocodazole for 2 hours. 10 μM brefeldin A (BFA) was added as the nocodazole was washed out. Shown are confocal micrographs of cells that were fixed at the indicated time points and decorated with anti-GM130 antibody. The size bars represent 20 μm. (B–C) Golgi motility in living NRK cells was quantified by incubation with NBD-C6 ceramide prior to nocodazole addition. 10 μM brefeldin A (B), or 50 μM BAPTA-AM (C) was added during the nocodazole wash out. A peripheral region of interest was defined for each cell and the change in peripheral NBD fluorescence was recorded for 25 minutes at 37°C. The mean fluorescence intensity within the region of interest is plotted as a function of time. The number of experiments is n=3 for (B and C). The standard error in each case is indicated by bars. (D) Shown are confocal micrographs of NRK cells transfected with pEGFP-C1, pEGFP-p23 (199–212), and pEGFP-p23(199–212, KK-AA). Cells expressing the GFP constructs are marked with an asterisk. The cells were pretreated for 2 hours with 20 μM nocodazole. Nocodazole was washed off, and the cells were incubated for 20 minutes before fixation and decoration with a mouse polyclonal antibody against the Golgi marker, GM130. The bar represents 10 μm. (E) The percentage of cells with juxtanuclear Golgi membranes at the 20-minute time point was determined after cells were scored in a blind manner. The average of 4 experiments is plotted. The bars indicate standard error. The percentage of cells displaying juxtanuclear Golgi is significantly increased in the presence of GFP-p23 (p<0.05).