Fig. 2.
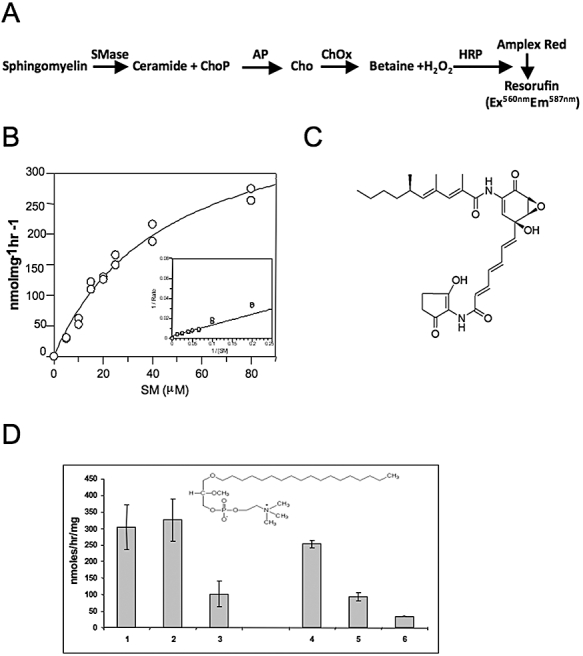
Activity of recombinant TbnSMase. A. Reaction catalysed by TbnSMase along with coupled the coupled Amplex red assay. AP, alkaline phosphatase; ChOx, choline oxidase; Cho-P, choline–phosphate; HRP, horseradish peroxidase. B. Determination of TbnSMase Michaelis-Menten constants for SM (inserts show Lineweaver–Burk plot). C. Structure of manumycin A, a commercially available nSMase inhibitor. D. Enzyme activity of TbnSMase in either washed bloodstream T. brucei membranes (lanes 1–3) or E. coli membranes expressing GST–TbnSMase (lanes 4–6), after pre-incubation with either nothing (lanes 1 and 4) or miltefosine (lanes 2 and 5) or edelfosine (lanes 3 and 6) in the presence of SM as substrate as described in Experimental procedures. Insert shows structure of edelfosine.
