Table 1.
Antiviral and cytotoxicity data of 13-38 and 40*
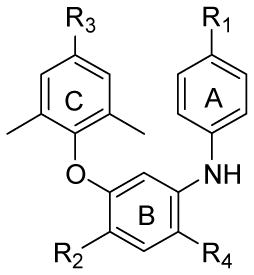
| Compound | R1 | R2 | R3 | R4 | HIV-1 IIIB in H9 cells |
||
|---|---|---|---|---|---|---|---|
| EC50a (μM) | CC50b (μM) | SIc | |||||
| 13 | OMe | NO2 | H | NO2 | 3.840 | >61.12 | >16 |
| 14 | OMe | NO2 | Me | NO2 | 2.990 | >59.10 | >20 |
| 15 | OMe | NO2 | Br | NO2 | 3.630 | 51.23 | 14 |
| 16 | Me | NO2 | Br | NO2 | 4.310 | 52.97 | 12 |
| 17 | Cl | NO2 | Br | NO2 | NA | — | — |
| 18 | NO2 | NO2 | Br | NO2 | >49.7 | >49.70 | >1 |
| 19 | CN | NO2 | Br | NO2 | 0.172 | >51.76 | >301 |
| 20 | CN | NO2 | H | NO2 | 0.545 | >61.88 | >113 |
| 21 | CN | NO2 | CN | NO2 | 4.190 | >58.28 | >14 |
| 22 | CN | NO2 | Me | NO2 | 0.280 | >59.81 | >214 |
| 23 | CN | NO2 | CHO | NO2 | 1.530 | 57.87 | 38 |
| 24 | CN | H | Br | NO2 | 0.317 | >57.08 | >180 |
| 25 | CN | H | H | NO2 | 3.147 | 69.64 | 22 |
| 26 | CN | H | CN | NO2 | 0.208 | >65.10 | >313 |
| 27 | CN | H | Me | NO2 | 0.067 | >67.02 | >1,000 |
| 28 | CN | H | CHO | NO2 | 2.190 | 14.20 | 6 |
| 29 | CN | H | Br | NH2 | 0.047 | 44.61 | 949 |
| 30 | CN | H | CN | NH2 | 0.070 | 49.77 | 711 |
| 31 | CN | H | Me | NH2 | 0.073 | 51.02 | 699 |
| 32 | CN | NH2 | Br | NH2 | 0.161 | 38.38 | 238 |
| 33 | CN | NH2 | H | NH2 | 3.226 | 53.20 | 16 |
| 34 | CN | NH2 | CN | NH2 | 0.030 | 50.95 | 1,698 |
| 35 | CN | NH2 | Me | NH2 | 0.070 | 50.00 | 714 |
| 36 | CN | NO2 | Br | NH2 | 0.016 | 45.47 | 2,842 |
| 37 | CN | NO2 | CN | NH2 | 0.003 | 62.66 | 20,887 |
| 38 | CN | NO2 | Me | NH2 | 0.062d | 32.22d | 520 |
| 40 | Hydrochloride salt of 36 | 0.012 | 36.42 | 3,035 | |||
| AZT | 0.052 | 1873 | 36,019 | ||||
Assays using H9 cells were performed at Panacos Pharmaceuticals, Inc., Gaithersburg, Maryland, USA. Results are averages of three independent assays. Standard deviations were not provided by Panacos.
Concentration of a compound that causes 50% inhibition of HIV-1 IIIB replication.
Concentration of a compound that causes cytotoxicity to 50% cells.
SI (selective index) = CC50/EC50.
Data from Duke University, USA. NA: not active.
