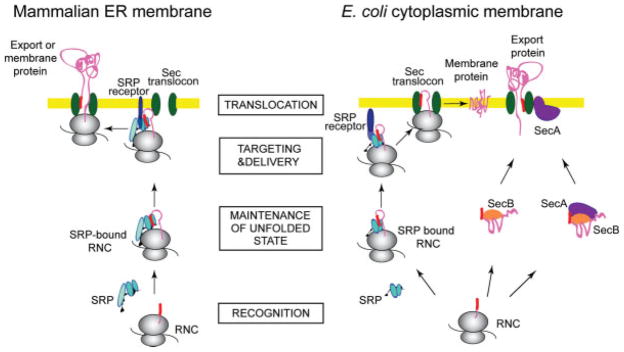
FIGURE 1.
Protein export to the endoplasmic reticulum (ER) and insertion into the ER membrane in eukaryotes, and to the plasma membrane or the periplasm in E. coli. SRP-mediated targeting: As a membrane protein (pink) is emerging from the ribosome (gray), SRP (light blue) recognizes and binds the signal sequence (red), and the entire nascent chain complex is targeted to the SRP receptor (dark blue) at the target membrane (yellow). In eukaryotes, translation is arrested or retarded at this step. The nascent protein is maintained in a conformation compatible with translocation. Upon interaction between the SRP and the SRP receptor, the ribosome nascent chain is delivered to the Sec translocon complex (green), which enables the nascent protein to cross or insert into the membrane. SRP is released and starts a new targeting cycle. SecA-mediated targeting: After a secretory protein is translated on the ribosome, the molecular chaperone SecB (orange) binds to the mature regions of the preprotein and keeps it in a conformation competent for translocation. This SecB-preprotein complex may be recognized by SecA (purple), which binds both the signal sequence and the mature part of the preprotein, either in its membrane-resident or its cytoplasmic form. The complex docks on the SecYEG translocon, and SecB is released. SecA undergoes major conformational changes as it inserts into the membrane and carries out its next roles as a “translocase,” facilitating translocation of the preprotein across the inner membrane via the translocon channel.