FIGURE 2.
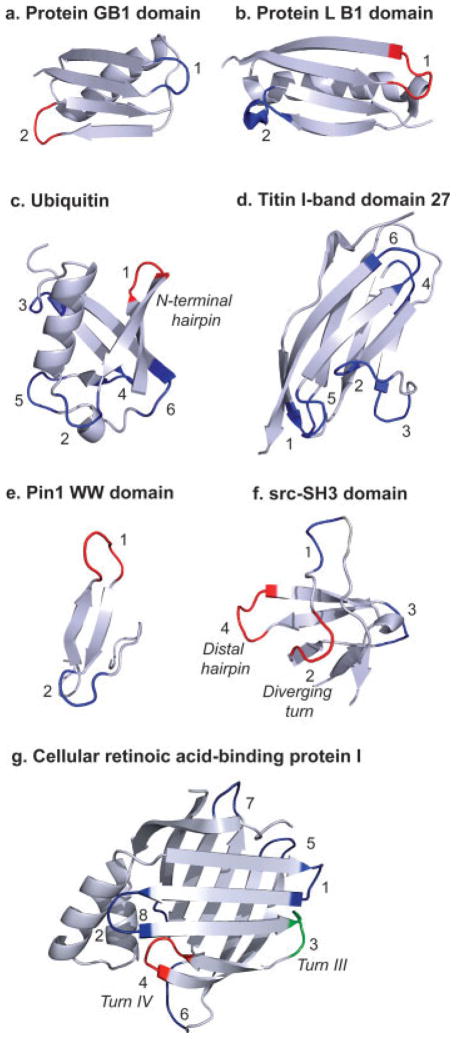
Proteins in which the role of turns in folding has been investigated: a, protein G B1 domain; b, Protein L B1 domain; c, ubiquitin; d, 27th domain of titin I-band; e, WW domains (the Pin1 WW domain is shown as an example); f, SH3 domains (the src-SH3 domain is shown as an example); and g, cellular retinoic acid-binding acid protein I (CRABP I). The turns for each protein/protein domain are numbered and are classified based on their structure. The PDB IDs for the proteins shown are listed in Table I. The turns colored red are those that play an active role in folding based on peptide models and protein engineering. All other predicted turns are colored dark blue. The third turn in CRABP I (labeled 3, and referred to as “Turn III” in the text and in the literature) is colored green. This turn has a high propensity to take up a native-like conformation as a peptide but is proposed to play a passive role in folding (see text).