Figure 1.
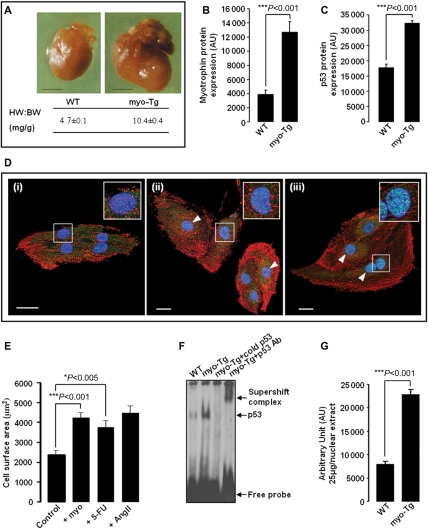
Myotrophin (myo)-induced heart failure (HF) in 36-week-old myo-Tg mice is associated with elevated expression of p53 and activation of p53 by myo, stimulating cardiac cell growth. (A) Overexpression of myo in the hearts of myo-Tg mice results in enlarged hearts and increased HW:BW ratio compared with age-matched wild-type (WT) mice during HF (>36 weeks) (black bar represents 10 mm); (B) A significant (P < 0.001) increase in myo protein in 36-week-old myo-Tg mice; (C) Elevated level of p53 protein in 36-week-old myo-Tg mice; (D) Confocal micrographs (×63) of neonatal rat cardiomyocytes immunostained for p53 (AlexaFluor®488-green/cyan), for sarcomeric α-actinin (AlexaFluor®568-red), and for nucleus (DAPI, blue). Neonatal rat cardiomyocytes [panel (i) is unstimulated] showed increased nuclear localization of p53 (white arrowhead) after treatment with myo (panel ii) and 5-FU (panel iii). The two-fold enlarged view of a single nucleus appears in the inset (white bar represents 20 µm); (E) Neonatal rat cardiomyocytes, stimulated with myo and 5-FU showing significant (Bonferroni's corrected P < 0.001 and P < 0.05, respectively) increase in cell surface area compared with untreated control cells. Ang II stimulation was performed as positive control. The data (mean ± SEM) for each group is an average of nine individual measurements; (F) EMSA showing increased activation of p53 in myo-Tg mice compared with WT mice; (G) Quantification of EMSA showing significant (P < 0.001) increase in p53 activation in myo-Tg mice compared with WT mice. Values obtained from three independent experiments are expressed as arbitrary units (AU). [Data for (A) and (B) were adapted from Sarkar et al.11 and Gupta et al.38].