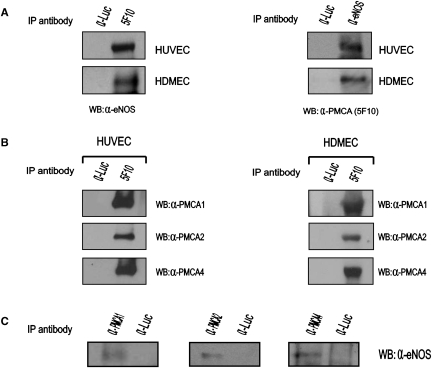
Figure 1.
Endogenous PMCA and eNOS interact in human endothelial cells. (A) eNOS and PMCA co-precipitate in endothelial cells. (Left panels) Protein lysates isolated from primary HUVEC or HDMEC were incubated with an anti-PMCA monoclonal antibody (5F10) and immunoprecipitated proteins analysed by western blot with an anti-eNOS rabbit polyclonal antibody. (Right panels) Endothelial protein lysates were immunoprecipitated with an anti-eNOS rabbit polyclonal antibody (α-eNOS) and immunoprecipitated proteins probed against the 5F10 anti-PMCA monoclonal antibody. (B) PMCA isoforms 1, 2, and 4 are expressed in endothelial cells. Protein lysates isolated from HUVEC or HDMEC were precipitated with an anti-PMCA monoclonal antibody (5F10) and precipitated proteins were probed with rabbit polyclonal antibodies recognizing specifically PMCA isoforms 1, 2, or 4. (C) Protein extracts isolated from HUVEC were immunoprecipitated with polyclonal antibodies recognizing specifically the isoforms 1 (α-PMCA1) or 2 (α-PMCA2) of PMCA. Immunoprecipitated proteins were probed with a mouse anti-eNOS antibody (Zymed). Likewise, protein extracts were precipitated with the JA9 anti-PMCA4 monoclonal antibody (α-PMCA4) and immunoprecipitated proteins subsequently probed with a rabbit anti-eNOS polyclonal antibody. In all cases, eNOS was present within the immunoprecipitated proteins, suggesting that PMCA 1, 2, and 4 isoforms interact with eNOS in endothelial cells. Immunoprecipitation with an irrelevant antibody against firefly luciferase (α-Luc) was included as a negative control in all immunoprecipitation experiments.