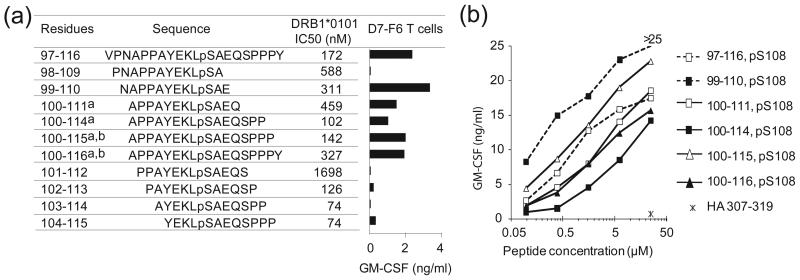
Figure 4.
Binding of phospho-MART-1 peptides to HLA-DR1 and recognition by CD4+ T cells. (a) Affinities of overlapping phosphorylated MART-1 peptides for HLA-DR1, as measured by 50% inhibitory concentrations (IC50), are shown. Synthetic phosphopeptides include four naturally expressed sequences isolated from cultured melanoma cells: a)1363-mel and b) 2048-mel. Bar graph demonstrates specific recognition of phospho-MART-1 peptides (15 μM) by the CD4+ T cell clone D7-F6. GM-CSF secretion from T cells was measured by ELISA; background secretion from T cells + APC + HA307-319 control peptide was <0.02 ng/ml. Similar results were obtained with IFNγ secretion, and using the parent T cell line D7. Results are representative of four separate experiments. (b) Recognition of titrated overlapping phospho-MART-1 peptides (0.1–25 μM) by the specific CD4+ T cell line D7. Background GM-CSF secretion from T cells + APC + HA307-319 control peptide (25 μM) was 0.7 ng/ml. Results are representative of three separate experiments. Similar results were observed with IFNγ secretion.