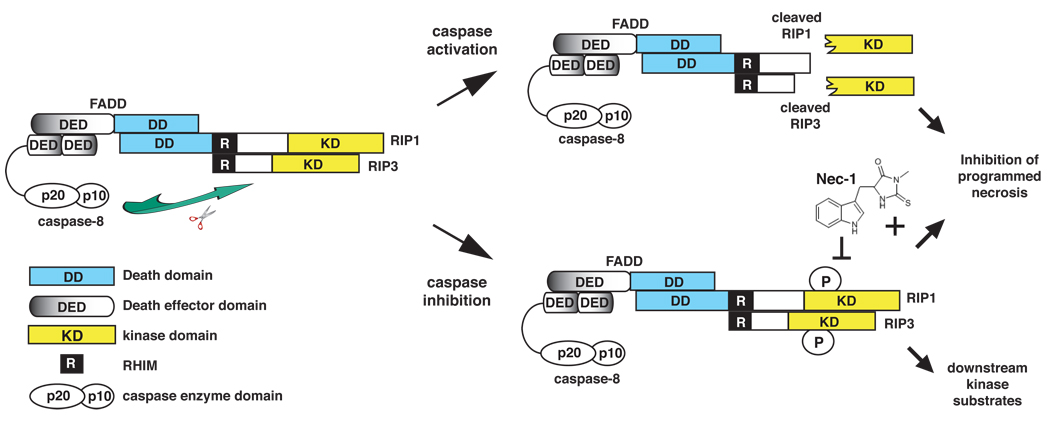
Figure 2. Caspase 8-mediated cleavage of RIP1 and RIP3 inhibits programmed necrosis.
The assembly of a cytoplasmic RIP1–RIP3 complex via the RHIM is critical for programmed necrosis. This complex is further stabilized by phosphorylation of RIP1 and RIP3. FADD and caspase 8 inhibit TNF-induced programmed necrosis by cleaving RIP1 (at D324) and RIP3 (at D333) within this complex. Cleavage results in the release of amino-terminal fragments containing the kinase domains, thereby preventing phosphorylation of RIP1, RIP3 and other possible downstream substrates. Necrostatin-1 (Nec-1), which inhibits RIP1 kinase activity, blocks programmed necrosis by preventing the stable association of the RIP1–RIP3 complex.