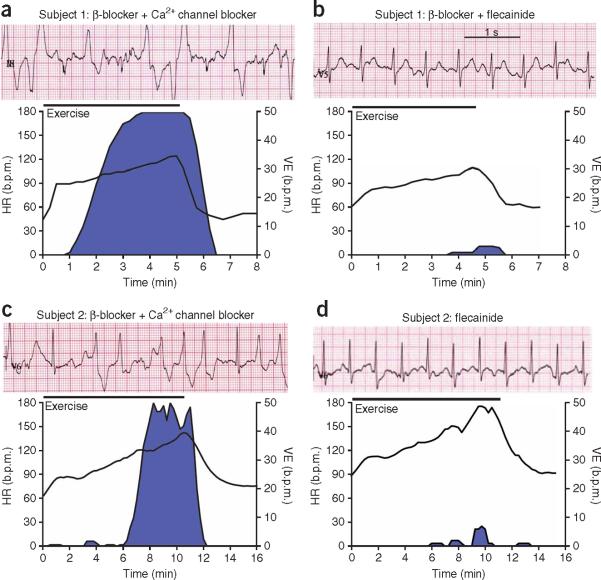
Figure 2.
Flecainide treatment prevents exercise-induced ventricular arrhythmia in two subjects with CPVT refractory to conventional drug therapy. (a–d) Effect of flecainide treatment in a 12-year-old boy (subject 1) with a CASQ2 mutation (a,b) and in a 36-year-old female (subject 2) with an RYR2 mutation (c,d). Top of each panel, representative ECGs recorded during maximum stress; bottom of each panel, heart rate and rate of ventricular extrasystoles during an exercise test. Blue area indicates arrhythmia burden. Exercise protocol and ventricular extrasystole analysis are as previously described4. Drug therapy during each exercise test: metroprolol 100 mg d−1 plus verapamil 120 mg d−1 (a), metroprolol 100 mg d−1 plus flecainide 150 mg d−1 for 7 weeks (b), bisoprolol 5 mg d−1 plus verapamil 240 mg d−1 (c) or flecainide 150 mg d−1 for 8 weeks (d). Experiments involving human subjects were reviewed and approved by the University of Amsterdam Academic Medical Center Institutional Medical Ethical Review Board. Both human subjects (or their parents) provided informed consent.