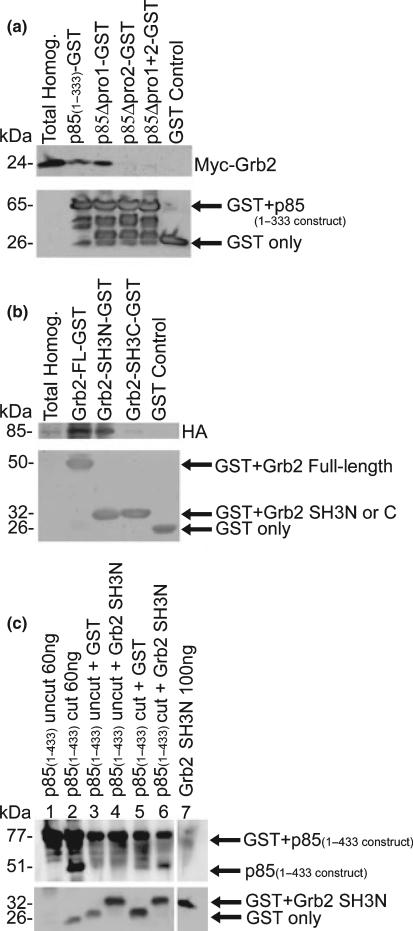
Fig. 6.
Pull-downs demonstrate that p85 directly binds to the SH3 domain of Grb2 via its second proline-rich region. (a) Full-length myc-tagged Grb2 was transfected into COS7 cells and pull-down assays were performed on protein homogenates with p85(1–333)-GST fusion proteins spanning both proline-rich regions [amino acids 1–333; GST-fusion protein containing p85Δpro1, p85Δpro2-GST, and p85Δpro1 + 2-GST; or GST only protein (negative control)]. Western blot analysis performed with a myc mAb (1 : 20 000) confirmed the presence of Grb2 in the total homogenates (b) COS7 cells were transfected with HA-tagged p85, and pull-down assays were performed on the protein homogenates using full-length or the N- or C-terminal SH3 domain of Grb2 fused to GST, or GST only protein (negative control). Western blot analysis performed with an HA mAb (1 : 1000) confirmed the presence of p85. (c) Pull-down assays were performed using either Grb2-SH3N-GST or GST only incubated with either thrombin cut p85(1–433), or uncut p85(1–433) GST. The p85(1–433) constructs span both proline-rich regions and the first SH2 domain of p85. The presence of p85 on the blot was detected using a pAb generated to a p85-GST fusion protein. The lower panels (a–c) show GST with or without the expressed fusion protein, using a GST mAb (DT-12, 1 : 100).