Figure 1.
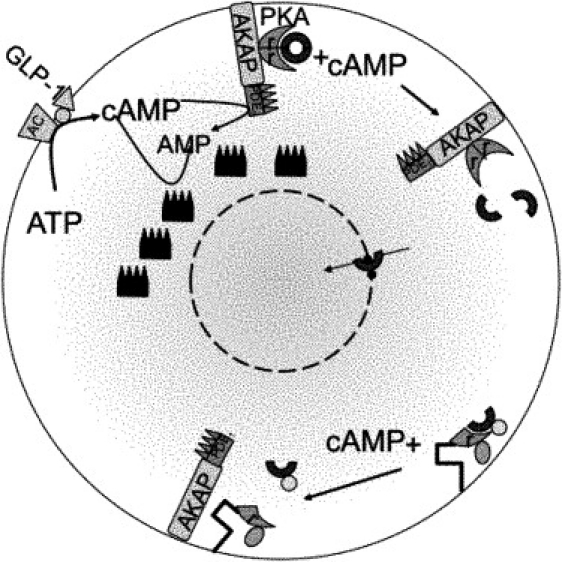
Cartoon of cAMP-dependent PKA nuclear translocation. GLP-1 activation of the GLP-1 receptor, a G-protein coupled receptor, triggers adenylyl cyclase (AC)-mediated production of cAMP from ATP. cAMP is degraded by various phosphodiesterase (PDE) isoforms. Binding to protein kinase A (PKA) anchored to the membrane through A-kinase-anchoring protein (AKAP) releases the catalytic subunits (cPKA) from the regulatory subunits (rPKA). cPKA can then enter the nucleus through nuclear pores. Dyachok et al. (7) labeled rPKA with cyan fluorescent protein (CFP) and cPKA with yellow fluorescent protein (YFP) and independently anchored labeled rPKA. Measuring with TIRF within 100 nm of the plasma membrane detects cPKA separation as a reduction in the YFP signal. YFP-tagged cPKA cannot enter the nucleus, so epifluorescence was used to measure cPKA penetration of the nuclear membrane when cAMP was raised. We hypothesize a rapidly buffering protein (small, dark blue dots) that delays cPKA entry into the nucleus.
