Abstract
Visual sensitivity decreases with age, and this presumably has an impact on face recognition. However, the relationship between aging in basic visual processing and in the sensory and cognitive mechanisms mediating face recognition is not well understood. Face detection, a foundational step in recognizing faces, relies primarily on sensory information. This study measured the ability to detect facial configuration and contrast detection in young (<40 years), middle–aged (40–59 years), and elderly adults (>59 years). Performance on both face detection and contrast detection was moderately degraded in the middle-aged group compared with the young group and was further degraded in elderly adults. Face detection was correlated strongly with contrast sensitivities, but only weakly with verbal IQ. The results suggest that face detection ability begins to reduce in early aging, and is associated with spatiotemporal visual processing.
Keywords: Aging, Contrast detection, Face recognition, Vision
FACE recognition ability declines with normal aging. Older adults are less accurate than younger adults at recognizing certain facial emotions (Calder et al., 2003; Ruffman, Henry, Livingstone, & Phillips, 2008) and discriminating facial identities (Cronin-Golomb, Gilmore, Neargarder, Morrison, & Laudate, 2007; Habak, Wilkinson, & Wilson, 2008). The capacity to process basic visual information such as contrast and motion also declines with age (Andersen & Ni, 2008; Billino, Bremmer, & Gegenfurtner, 2008; Glass, 2007; Owsley, Sekuler, & Siemsen, 1983). Recent research exploring the relationship between basic visual processing and daily living activities in aging populations suggests a close relationship between visual decline and decline in daily living activities (Berger & Porell, 2008; Dunne, Neargarder, Cipolloni, & Cronin-Golomb, 2004; Owsley, Ball, Sloane, Roenker, & Bruni, 1991; Scilley et al., 2002).
As social animals, people interact with peers on a daily basis, during which face recognition is an essential perceptual function. Face detection, the process by which a face is identified as such, represents the putative first stage in a cascade of functional processes for face recognition. This perceptual capacity requires not only the detection of visual features presented in faces but also the integration of local signals of facial features into a holistic representation (Bruce & Young, 1986; Farah, Wilson, Drain, & Tanaka, 1998; Tsao & Livingstone, 2008).
Whether and how performance in face detection is affected by age-related decline in basic visual processing on one hand, and by age-related cognitive decline on the other, is a question that has not been systematically investigated. Slower speed of processing in elderly adults (Salthouse, 1996) appears to have broad effects on perception and cognition, and it seems likely that the processing and integration of local features during face detection may be one such process that is affected. If so, other face-specific processes that depend on face detection (Johnson, 2005) would also become less efficient. To our knowledge, only one study has reported that elderly adults are less sensitive than young adults at detecting a face (Owsley, Sekuler, & Boldt, 1981). However, the precise course of aging in face detection, and the role that aging of sensory and cognitive mechanisms play in that process, is still unclear. Face recognition comprises a series of component processes spanning sensory, cognitive, and affective domains (Bruce & Young, 1986). Aging in facial emotion discrimination, for example, appears to relate to changes in cognitive, rather than sensory, processes (Orgeta & Phillips, 2008). However, face detection is more sensory in nature, as the processing of more sophisticated aspects of facial information (e.g., identification and memory of individual faces) is not required at this stage of face recognition. As such, the perceptual capacity of face detection may be more closely linked to age-related changes in sensory, rather than cognitive, domains.
The present study investigated face detection and basic visual processing (contrast detection) in healthy individuals whose ages ranged across the adult life span (20–80 years old). Psychophysical methods were used to measure the ability to detect simple face images as well as visual contrast sensitivity to a range of spatial and temporal frequencies. The sensitivity to detect a range of spatial and temporal frequencies provides an index for basic visual processing. This basic visual signal is important for face detection, as both low– and high–spatial frequency components can affect cortical responses to face images (Halit, de Haan, Schyns, & Johnson, 2006). Performance in visual contrast detection, face detection, and verbal IQ were compared in and across young, middle-aged, and elderly groups. We hypothesized that performance in both perceptual tasks would decline with age. In addition, we hypothesized that face detection performance would be related to contrast detection performance, but not related to verbal IQ, throughout the adult life span because face detection relies primarily on visual signals.
METHODS
Participants
Sixty-two individuals, ranging from 20 to 80 years old, participated in the study. There were an approximately equal number of people in each of the three age groups: young (20–39, M age 27.2 years, SD = 5.2), middle-aged (40–59, M age 51.3 years, SD = 5.0), and elderly (60–80, M age 69.6 years, SD = 5.7). The young group consisted of 13 women and 7 men, with an average education of 16.5 years (SD = 2.1). The middle-aged group consisted of 10 women and 9 men, with an average education of 15.5 (2.8). The elderly group consisted of 15 women and 8 men, and had an average education of 15.4 years (2.4). All subjects had a verbal IQ greater than 90, visual acuity of at least 20/40, no recent history of drug or alcohol abuse (i.e., within the past 6 months), no history of organic brain disease, and no history of psychiatric illness. Participants were community-dwelling residents of the Boston area, recruited through advertisements posted in the local community.
Stimuli and Procedures
Face detection.—
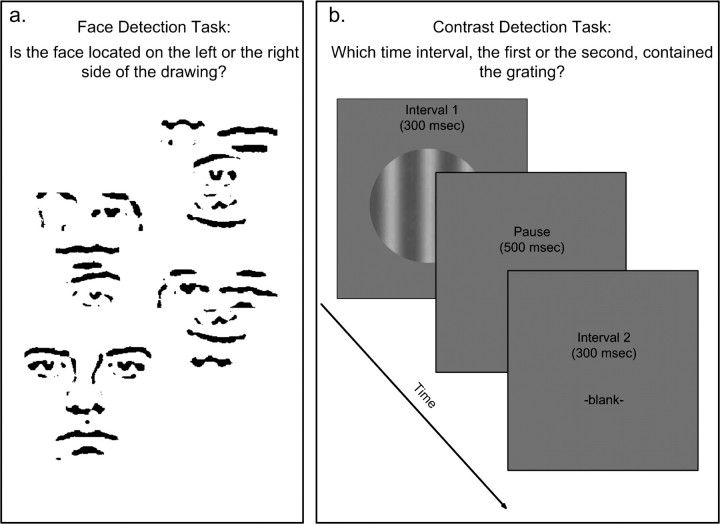
The target for face detection was a face image composed of line segments and was embedded in a cluster of larger, scrambled line segments, as depicted in Figure 1a. Compared with other face images (such as photographs), this simple face image contains primarily facial configuration, not other information such as skin color or shading, which is not specifically associated with faces. The position of the target could be on the left side or the right side of the larger drawing. The drawings were displayed on a computer screen for 13, 26, 52, or 104 ms.
Figure 1.
Illustration of stimuli and task procedures. (a) For face detection, subjects indicated on which side, the left or the right, a line-drawn face embedded within a larger drawing was located. Images were presented briefly (13–104 ms). (b) For contrast detection, subjects indicated which of two time intervals contained a sinusoidal grating on the computer screen. Gratings were either temporally modulated or static, with varying spatial and temporal frequencies.
Subjects' task was to judge whether the target was located on the left or on the right side of the drawing (a two-alternative forced choice). For each presentation, subjects gave their judgments by pressing one of two designated keys on a keyboard. The stimulus presentations were blocked into sessions according to presentation time (13, 26, 52, and 104 ms). Each block contained 42 trials. The purpose of the blocked design was to provide the stimuli with equal presentation time in each session to avoid confusion in naive subjects. The order of the sessions was randomized across subjects. Performance was measured by the proportion of correct responses in detecting the location of the face images.
Contrast detection.—
The target for contrast detection was a grating that was spatially modulated with a sinusoidal waveform (Figure 1b). The grating was presented on a computer monitor and had an average luminance of 35 cd/m2. Each trial included two stimulus presentations, one containing the target and the other containing a blank screen with the same average luminance level as the target. The target and the blank screen were each displayed for 300 ms, with a 500-ms interval in between. Subjects’ task was to judge which of the two presentations, the first or the second, contained the target. Static contrast detection included four spatial frequencies: 0.5, 1, 2, and 4 cycles per degree (c/d). Dynamic contrast detection included four temporal frequencies: 1, 2, 4, and 8 Hz, all with spatial frequency of 0.5 c/d. The performance was indexed by contrast detection thresholds, which were determined by a two-alternative forced-choice staircase procedure. The threshold represents the minimum contrast level where performance reached 79% accuracy (Levitt, 1972). Thresholds for each stimulus condition were converted to contrast sensitivity (log of 1/threshold).
The participants practiced the task at a high contrast level before data collection began, for as long as was necessary for them to perform accurately.
Verbal IQ.—
The Wechsler Adult Intelligence Scale–Revised (WAIS-R; verbal components) was administered (Wechsler, 1981). The verbal component of the test includes an assessment of vocabulary, general knowledge, arithmetic, digit span, comprehension, and similarities. In addition to the standard scaled scores, which adjust for age, the raw scores for each subject were also indexed. The use of this standard rubric allows for evaluation and comparison of general intelligence and cognition among subjects irrespective of age.
RESULTS
Face Detection
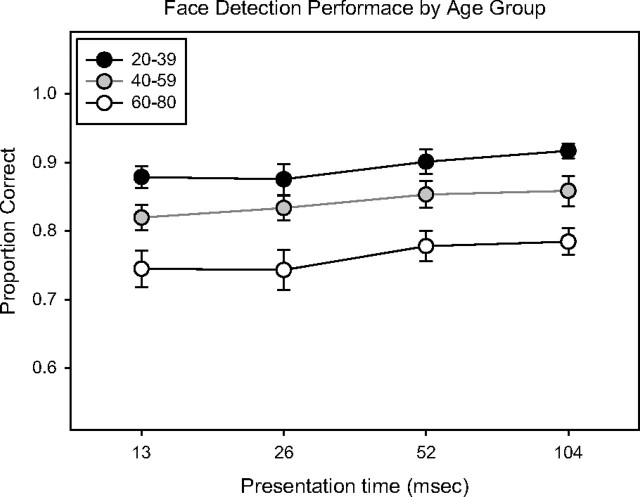
Accuracy was lowest in older adults (M = 77.9%, SD = 9.7%), and lower in middle-aged adults (M = 85.3%, SD = 6.6%) than in younger adults (M = 90.4%, SD = 6.0%) (Figure 2). An analysis of variance (ANOVA) with age group and presentation time as factors showed a significant effect for age group (F = 49.7, p < .001) and presentation time (F = 2.8, p = .04), but no interaction between the two (F = 0.16, ns). Comparing only middle-aged and young adults, the analysis showed the same pattern, with younger subjects performing better (F = 21.5, p < .001). Comparing only the middle-aged and elderly groups, the elderly group performed worse (F = 29.7, p < .001). There was no interaction between age group and presentation time in any of the aforementioned analyses. The effect sizes (ESs) between the young and middle-aged, and middle-aged and older groups were 0.80 and 1.60, respectively, averaging across presentation times.
Figure 2.
Summary of face detection performance in the three age groups. The ordinate represents accuracy, reported as the proportion correct. The abscissa is the presentation time of the stimulus. Error bars represent 1 SE.
Contrast Detection
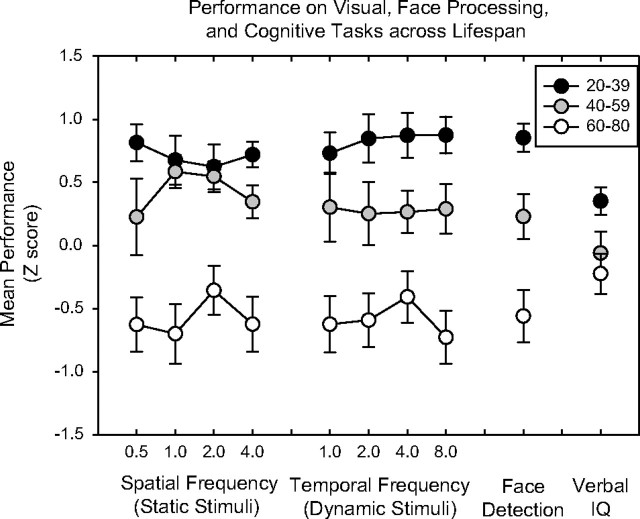
Relative to the youngest age group, contrast sensitivity was lower in the middle-aged and elderly groups for all spatial and temporal frequencies tested (Figure 3). For static stimuli, the mean sensitivities (averaged across spatial frequencies) for young, middle-aged, and elderly adults were 2.62 (0.13), 2.55 (0.13), and 2.29 (0.23), respectively. A two-way ANOVA on age group and spatial frequency showed significant effects for age group (F = 45.6, p < .001) and spatial frequency (F = 34.0, p < .001). For dynamic stimuli, the mean sensitivities (averaged across temporal frequencies) were 2.84 (0.12), 2.71 (0.16), and 2.52 (0.19) for the young, middle-aged, and elderly groups, respectively. ANOVA on temporal frequency and age group showed significant effects for age group (F = 46.8, p < .001) and temporal frequency (F = 8.0, p < .001). When comparing only young and middle-aged groups, the group difference remained significant for the static (F = 5.4, p = .02) and dynamic (F = 15.2, p < .001) conditions, with middle-aged adults showing reduced sensitivities to both types of visual stimuli. Comparing the middle-aged and elderly groups, the differences were also significant for the static (F = 38.9, p < .001) and dynamic (F = 29.4, p < .001) conditions. The ES for the static conditions, averaging across spatial frequencies, was 0.27 between the young and middle-aged group, and 1.50 between middle-aged and elderly group. For the dynamic conditions, the ESs, averaging across temporal frequencies, were 0.57 between the young and middle-aged and 1.54 between the middle-aged and older group.
Figure 3.
Summary of visual task performance and verbal IQ scores for the three age groups. To compare across tasks, contrast sensitivities (log of 1/threshold—the measure for contrast detection), averaged accuracies across the presentation times (the measure for face detection), and raw IQ scores (without adjusting for age) were converted to z scores. Each data point represents the mean z scores for a particular task condition. The higher a z score, the better the performance is relative to the other age groups. The error bars represents 1 SE.
Verbal IQ
The mean standardized verbal IQs for the young, middle-aged, and elderly groups were 115.9 (SD = 9.8), 111.8 (9.9), and 113.9 (10.3), respectively. An ANOVA across the three age groups showed no difference in the cognitive measure (F = 0.83, ns), indicating that with respect to individuals’ ages, all participants performed similarly. As indicated in the Methods, the raw WAIS measurements, which do not correct for age, were also used. These raw IQ scores were converted to z scores for comparison with visual performance (Figure 3). For these scores, ANOVA showed a significant difference among the three age groups (F = 3.91, p = .03), indicating that, overall, cognitive performance was worse in the two older age groups.
Regression Analysis of Visual and Cognitive Tasks Across Age
Linear regression revealed negative slopes for visual and cognitive task performance as a function of age. For face detection, the slope of the face performance across the age range (20–80 years) was −0.33 (z scores per decade) (p < .001). For static contrast detection, slopes ranged from −0.25 to −0.37 (p ≤ .001), and for dynamic conditions, the slopes ranged from −0.33 to −0.41 (p < .001). For raw IQ scores, the slope was smaller—only −0.14 (p = .005).
Multiple regression analysis was also performed to determine which variables—age, contrast detection (at 0 Hz, 0.5 c/d, which was the condition most closely related to face detection in correlation analysis; Figure 4), IQ, or all—predicted face detection performance. Cumulatively, these three variables predicted 44% of the variability in face detection performance (averaged across the four presentation times). Of these variables, only contrast sensitivity (t = 6.09, p = .019) and age (t = 4.88, p = .034) were significant predictors. Verbal IQ was not a significant predictor (t = 0.131, ns).
Figure 4.
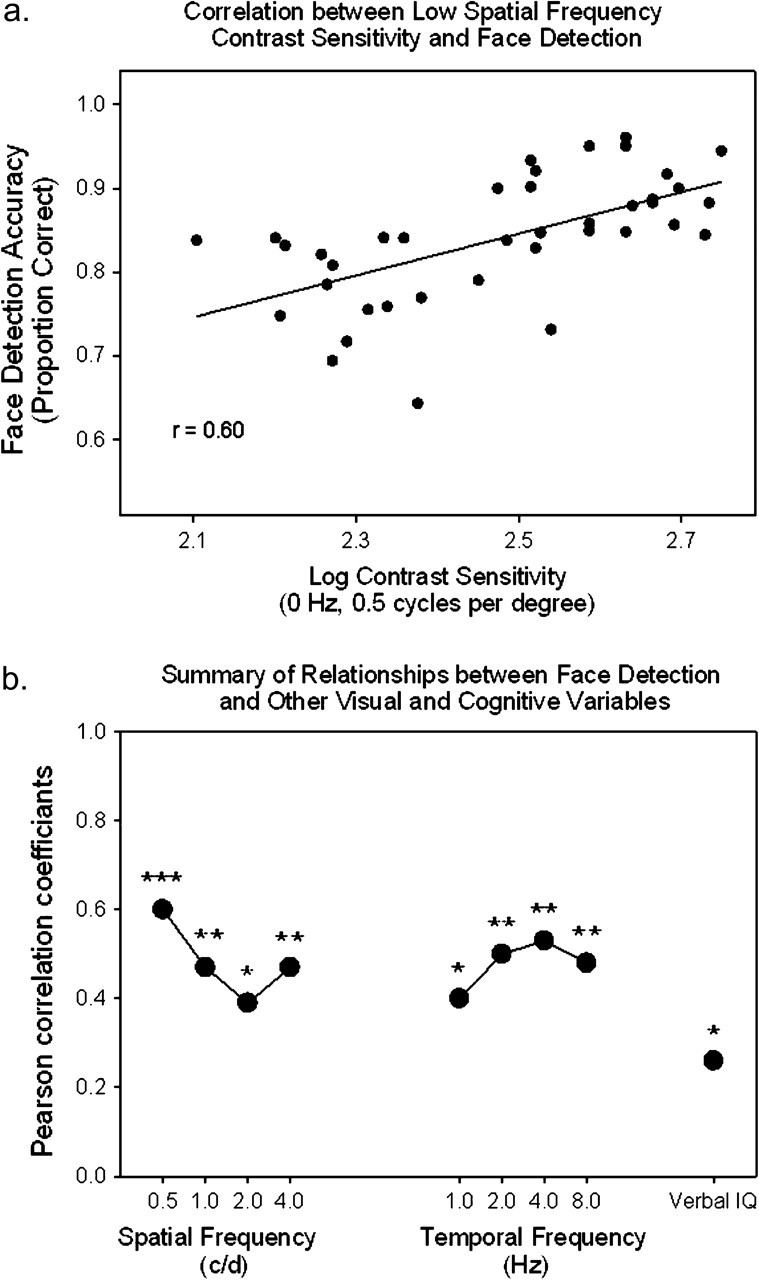
(a) Correlation between performance accuracy in face detection and contrast sensitivity at 0 Hz and 0.5 c/d. (b) Correlations between face detection performance and contrast sensitivity at each spatial and temporal frequency and between face detection performance and verbal IQ. The four conditions grouped under “spatial frequency” represent correlation values for performance under the static contrast condition (i.e., a temporal frequency of 0 Hz), and the values are in cycles per degree (c/d). The four conditions grouped under “temporal frequency” all have a spatial frequency of 0.5 c/d and were drifting at the specified temporal frequencies listed in Hertz (Hz). *p < .05. **p < .01. ***p < .001.
Comparison of Face Detection With Visual and Cognitive Variables
Face detection performance, measured as averaged accuracy across the four presentation times, was highly and significantly correlated with contrast sensitivities at all spatial and temporal frequencies (.46 < r < .61, p < .01), with two exceptions (2 c/d and 0 Hz, and 0.5 c/d and 1 Hz), where the correlation was also significant, but to a lesser degree (r = .39 and .40, p < .05) (Figure 4a). Performance on face detection was only modestly correlated with IQ (r = .26, p = .049) (Figure 4b). Contrast sensitivities were not correlated with IQ (r < .23, ns).
DISCUSSION
Face detection performance and contrast sensitivity were both degraded in elderly adults as compared with younger adults, as expected. Not only elderly but also middle-aged adults showed degraded performance relative to young subjects in face detection and contrast sensitivity, suggesting that these perceptual capacities are vulnerable to early aging. Face detection was strongly related to contrast detection across the adult life span, as expected, whereas verbal IQ showed only a modest correlation with this perceptual capacity.
Face Processing, Visual Processing, and Aging
Elderly adults show reduced ability in identifying and recalling facial emotions and identities (D'Argembeau & van der Linden, 2004; Habak et al., 2008; Orgeta & Phillips, 2008; Ruffman et al., 2008). The results of the present study add to this literature by showing degraded face detection in middle-aged and older adults. Performance on this ability was strongly correlated with performance on basic visual detection tasks, especially with the static, low–spatial frequency stimuli. This latter relationship is consistent with literature showing that low–spatial frequency information is particularly important for face detection (Halit et al., 2006). It may well be that when the processing of low–spatial frequency information is compromised, as in aging populations, not only basic visual detection but also face detection is adversely affected.
The relationships between reduced face detection ability in aged individuals and visual processing, as well as other aspects of face recognition, are still not clear. In the realm of emotion processing, it has been found that recognition of negative emotions (anger, fear, sadness, and disgust) in elderly adults is related to their visual scanning patterns: for example, older adults tend to look at the lower halves of faces (i.e., the mouth) more than the upper halves (i.e., the eyes) (Sullivan, Ruffman, & Hutton, 2007; Wong, Cronin-Golomb, & Neargarder, 2005). The brief presentation of the images used in this study (13–104 ms) did not allow for eye movement during face detection. Therefore, declining face detection performance in normal aging is independent of the information processing stage where visual scanning is implemented. In contrast, although face detection is presumably an “all-or-none” perceptual capacity (the face is detected or not), the way in which it is detected could have an impact on other downstream aspects of the face processing by altering the speeds of initiation and efficiencies of additional face-related processes. This idea is consistent with the processing speed theory of age-related decline in cognition (Salthouse, 1996). As proposed by Salthouse, slower processing in earlier stages (in this case face detection) may have deleterious effects on later stages (e.g., expression recognition). Interestingly, face detection performance has been linked to social functioning scores in healthy adults (Chen, Norton, McBain, Ongur, & Heckers, in press).
One limitation of the present study is that the relationship between face detection and visual detection were characterized based on correlation analysis. However, the regression analysis indicates that age and contrast sensitivity are two distinct variables that affect face detection performance throughout the life span and cannot be entirely explained in terms of each other. Future studies should compare performance in face emotion recognition with face detection and manipulate the saliency of visual features in different parts of faces to elucidate the specific mechanisms underlying the decline in face recognition.
Early Aging in Face Detection and Basic Visual Detection
Understanding and characterizing face detection and basic visual detection throughout the adult life span is important from both biological and behavioral perspectives. Recent genetic work suggests that biological aging begins earlier than previously thought; for example, genetic disrepair in the frontal cortex seems to begin in persons’ 40s (Lu et al., 2004). Some behavioral results in the visual domain are consistent with early mental aging. For example, contrast sensitivity (for certain spatial frequencies) and motion discrimination seem to decline starting in persons’ 40s and 50s (Bidwell, Holzman, & Chen, 2006; Glass, 2007). The present study showed that face detection performance is degraded not only in elderly adults, as found previously (Owsley et al., 1981), but also in middle-aged individuals. The result from the middle-aged group indicates that the ability to detect a face is sensitive to early aging processes, despite many cognitive abilities remaining unaffected in this age range. The way in which perceptual decline of face information interacts with declining cognition in extreme old age should also be characterized in future studies.
Poor performance in contrast detection in middle-aged individuals, as compared with younger individuals, appears to be selective to certain spatial and temporal frequencies. For the static stimuli, the middle-aged adults group had lower mean sensitivities for the lowest and the highest spatial frequency conditions, indicating that within this age range the capacity of contrast detection starts to attenuate. Interestingly, the middle-aged group showed performance degradation relative to the young group to a greater extent for temporally modulated stimuli (ES = 0.57) than for static stimuli (ES = 0.27) (Figure 3). This difference suggests that the processing of dynamic stimuli is more sensitive to early aging. Decline in dynamic contrast detection in the middle-aged group (as compared with the younger group) is consistent with previous studies showing early aging with respect to motion processing (Bidwell et al., 2006; Snowden & Kavanagh, 2006).
Aging of the Brain Systems Involved in Face and Visual Detection
When processing facial emotion information, the aging brain appears to shift activity from perceptual centers in the posterior cortex to the anterior cortex (Fischer et al., 2005; Gunning-Dixon et al., 2003). This result suggests that vision-dependent behaviors in elderly adults rely less on sensory processing, which is compromised by aging, and more on cognitive processing, which may be affected at a relatively more gradual rate. Face detection appears to be more associated with contrast sensitivity, which is affected during early aging, and less associated with general cognition (i.e., verbal IQ). Therefore, the degraded face detection performance in the older groups, starting in the middle-age range, is consistent with the aforementioned hypothesis because face detection relies directly on sensory responses and may not be able to call upon cognition when sensory processes fail. However, it should be noted that the cognitive measure used in this study, the verbal portions of the WAIS-R, provides only a basic index of certain cognitive capacities (short-term memory, categorization, knowledge recall, etc.). Future studies should investigate whether other cognitive capacities such as processing speed, or other aspects of memory, are related to the decline in face detection performance.
Aging in the visual cortex significantly affects temporal processing (Mendelson & Wells, 2002). Neural units in the visual cortex of aged animals are less responsive to fast-moving visual stimuli (i.e., stimuli of higher temporal frequency) compared with those in younger animals. This suggests a decreased sensitivity to certain types of visual stimuli as a result of decreased response in the early stages of visual processing (e.g., that occurring in the striate cortex). Such decreased sensitivity likely plays a significant role in the age-related decline in dynamic contrast detection, as found in this and other perceptual studies.
Face Processing, Visual Processing, and Daily Living
Understanding the connection between visual decline and degraded daily living in normal aging may yield insights that will allow for improved daily living of elderly adults. Our data from the linear regression analysis indicate substantial declines in face detection and contrast detection with age but relatively shallow decline in verbal IQ. The steep rates of performance decline across the three age groups suggest that increasing the saliency of sensory signals may be helpful to compensate for deteriorating daily functions associated with visual aging. Some cognitive training interventions have been shown to reduce difficulty for elderly adults in daily living activities (Willis et al., 2006). Interventions focused on age-related decline in perceptual processing may also be effective. One possible way to do this would be to strengthen the visual signals of objects being used by older adults (e.g., make them brighter or of a higher contrast). Recent studies have shown that providing enhanced visual display is a promising direction because experimentally strengthened visual signals can improve cognitive and daily activity performance in age-related neurodegenerative diseases (Cronin-Golomb et al., 2007; Dunne et al., 2004). Another possible approach would be to improve sensory signals by retraining aging adults through perceptual learning, with which sensitivity to specific aspects of stimuli can be increased. Future studies should focus on identifying such age-sensitive and daily life–relevant processes that are accessible to perception-based intervention.
FUNDING
This work was supported in part by a grant from the National Institute on Aging through Harvard School of Public Health.
Acknowledgments
We thank Ken Nakayama, Grace Masters, Vanessa Lee, and Jejoong Kim for their help on the paper.
References
- Andersen GJ, Ni R. Aging and visual processing: Declines in spatial not temporal integration. Vision Research. 2008;48:109–118. doi: 10.1016/j.visres.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Berger S, Porell F. The association between low vision and function. Journal of Aging and Health. 2008;24:24. doi: 10.1177/0898264308317534. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Holzman PS, Chen Y. Aging and visual motion discrimination in normal adults and schizophrenia patients. Psychiatry Research. 2006;145:1–8. doi: 10.1016/j.psychres.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billino J, Bremmer F, Gegenfurtner KR. Differential aging of motion processing mechanisms: Evidence against general perceptual decline. Vision Research. 2008;48:1254–1261. doi: 10.1016/j.visres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77(Pt. 3):305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton D, McBain R, Ongur D, Heckers S. Visual and cognitive processing of face information in schizophrenia: Detection, discrimination and working memory. Schizophrenia Research. 2009;107:92–98. doi: 10.1016/j.schres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A, Gilmore GC, Neargarder S, Morrison SR, Laudate TM. Enhanced stimulus strength improves visual cognition in aging and Alzheimer's disease. Cortex. 2007;43:952–966. doi: 10.1016/s0010-9452(08)70693-2. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, van der Linden M. Identity but not expression memory for unfamiliar faces is affected by ageing. Memory. 2004;12:644–654. doi: 10.1080/09658210344000198. [DOI] [PubMed] [Google Scholar]
- Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A. Visual contrast enhances food and liquid intake in advanced Alzheimer's disease. Clinical Nutrition. 2004;23:533–538. doi: 10.1016/j.clnu.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neuroscience Letters. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Glass JM. Visual function and cognitive aging: Differential role of contrast sensitivity in verbal versus spatial tasks. Psychological Aging. 2007;22:233–238. doi: 10.1037/0882-7974.22.2.233. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformations that link facial identity across views. Vision Research. 2008;48:9–15. doi: 10.1016/j.visres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halit H, de Haan M, Schyns PG, Johnson MH. Is high-spatial frequency information used in the early stages of face detection? Brain Research. 2006;1117:154–161. doi: 10.1016/j.brainres.2006.07.059. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Levitt D. The transformed up-down methods. Journal of the Acoustical Society of America. 1972;10:465–479. [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Wells EF. Age-related changes in the visual cortex. Vision Research. 2002;42:695–703. doi: 10.1016/s0042-6989(01)00307-8. [DOI] [PubMed] [Google Scholar]
- Orgeta V, Phillips LH. Effects of age and emotional intensity on the recognition of facial emotion. Experimental Aging Research. 2008;34:63–79. doi: 10.1080/03610730701762047. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychological Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: Face perception. Investigative Ophthalmology and Visual Science. 1981;21:362–365. [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109:1235–1242. doi: 10.1016/s0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Ruffman T, Hutton SB. Age differences in emotion recognition skills and the visual scanning of emotion faces. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:P53–60. doi: 10.1093/geronb/62.1.p53. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annual Review of Neuroscience. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the adult intelligence scale–revised. New York: Psychological Corp; 1981. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B, Cronin-Golomb A, Neargarder S. Patterns of visual scanning as predictors of emotion identification in normal aging. Neuropsychology. 2005;19:739–749. doi: 10.1037/0894-4105.19.6.739. [DOI] [PubMed] [Google Scholar]