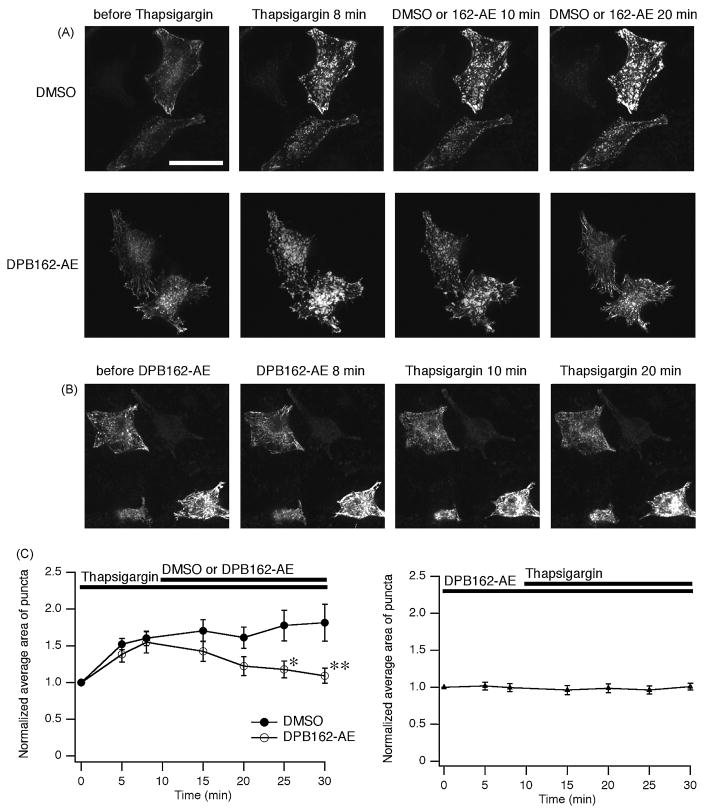
Fig. 6.
DPB162-AE inhibits thapsigargin-induced puncta formation of STIM1. (A) HeLa cells were transfected with EGFP-STIM1 to visualize their localization. In the resting state STIM1 proteins distribute in a radial and sparse manner throughout the cell (“before Thapsigargin”). Cells were treated with 1 μM of thapsigargin for 10 minutes then 3 μM of DPB162-AE or DMSO (for negative control) was added. After 8 minutes treatment with thapsigargin causes translocation of STIM1 into puncta (“Thapsigargin 8 min”). Following application of DMSO did not interfere with cluster formation (upper panels). Clustered EGFP-STIM1 dispersed upon following application of DPB162-AE (lower panels). (B) Preincubation with 3 μM of DPB162-AE for 10 minutes prevents the puncta formation induced by thapsigargin treatment. Twenty minutes of thapsigargin treatment (1 μM) failed to induce distinct clustered structures as observed in A (“Thapsigargin 20 min”). (C) Fluorescence puncta were identified and analyzed for the average area. Store depletion by thapsigargin treatment increased average area of puncta while 20 minutes incubation with 3 μM of DPB162-AE reversed the clustering almost to the baseline level (left panel). The effect of DPB162-AE on the average area of puncta was significant compared with DMSO negative control (*P < 0.02 at 15 minutes after DPB162-AE applied, **P < 0.001 at 20 minutes after DPB162-AE applied, t-test. n = 5 and 9 for DMSO and DPB162-AE, respectively). In contrast, HeLa cells pretreated with DPB162-AE did not show clear increase in average area of clusters (right panel, n = 17). Data points represent mean ± SEM. Scale bar: 50 μm.