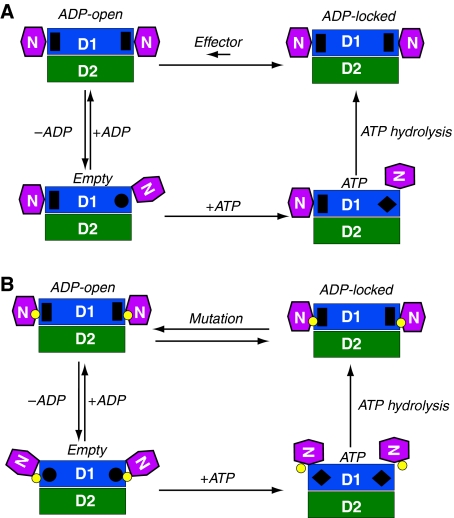
Figure 5.
Mechanistic model for the control of N-domain movement and implication for IBMPFD. Schematic diagram for the control of the N-domain conformation in (A) the wild-type and (B) IBMPFD mutant p97 N–D1 fragment. The N-, D1-, and D2-domains are in magenta, blue, and green, respectively, and as labelled. The small yellow circles between N- and D1-domains represent positions of mutations. Four states are defined for each nucleotide-binding site in D1: Empty state, ATP state, ADP-locked, and ADP-open, as labelled. Each protomer is assumed to operate independently. The stimuli for changes in D1 nucleotide state may come either from the N-domain or from ATP hydrolysis of the D2-domain. In the ADP-locked state, the N-domains are in Down-conformation with a pre-bound ADP shown as a black rectangle. This ADP-locked state has been observed crystallographically in wild-type p97. In the ATP state, the N-domains of hexameric wild-type p97 could be either in an Up-conformation with bound ATP shown as a black diamond or in a Down-conformation in an ADP-locked state, whereas the N-domains of mutants adopt only the Up-conformation as observed in this study. On the basis of available structural and biochemical information, we introduce two additional conformational states. The Empty state has the N-domain conformation undefined and the nucleotide-binding site shown as a black circle. The ADP-open state also has an N-domain conformation similar to that of Down-conformation as determined by the crystal structure of R155H mutant with bound ADP, which is also shown as a black rectangle. Bound ADP can only be exchanged through the ADP-open state. In wild-type p97, the equilibration between ADP-open and ADP-locked favours heavily the latter and is presumably regulated by effectors such as p47 or ATP hydrolysis in D2-domain. In IBMPFD mutants, the tight control between ADP-open and ADP-locked is disrupted and the equilibration is now favouring the ADP-open state.