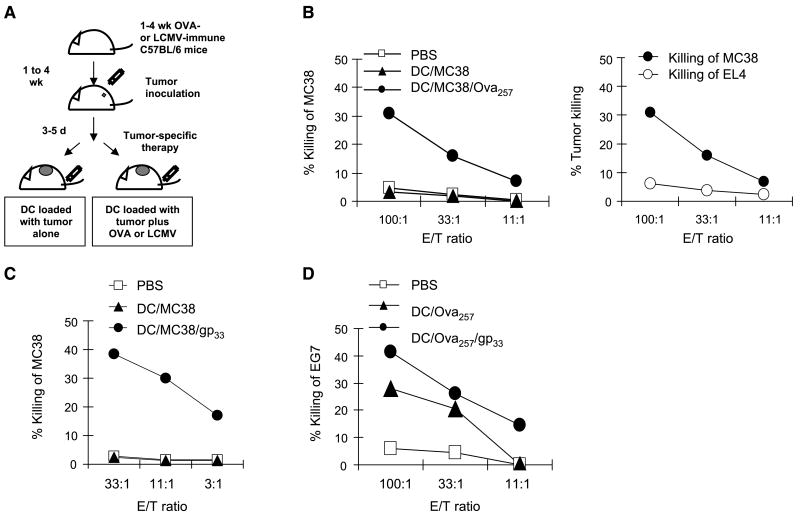
Figure 2.
Memory CD8+ T cells support the dendritic cell–mediated induction of tumor-specific CTL responses. A, schema of the experimental protocol. B to D, heterologous memory-type CD8+ T cells support the induction of tumor-specific CTLs by dendritic cell–based cancer vaccines. B, OVA257–264–specific CD8+ T cells support the induction of CTLs specific for MC38 adenocarcinoma. Mice carrying memory-type OVA257–264–specific CD8+ T-cell responses and inoculated with MC38 tumor (day 0) were treated s.c. (day 3) with dendritic cells loaded with MC38 lysate alone or with OVA257–264 as tumor-unrelated heterologous helper epitope. Left, induction of CTL activity in the spleens of the differentially treated mice. Right, comparison of CTL activity of the splenocytes from the dendritic cell/MC38 lysate/LCMVgp33–41–treated mice against the vaccine-relevant (MC38) and irrelevant (EL4) targets. Data from one of two experiments that yielded similar results. C, memory-type LCMVgp33–41–specific CD8+ T cells support the induction of MC38 adenocarcinoma–specific CTLs. Tumor-bearing mice with memory-type LCMVgp33–41–specific CD8+ T-cell responses were injected s.c. with dendritic cells loaded with MC38 tumor lysate, alone or with LCMVgp33–41 as a heterologous helper epitope. Data from one of three independent experiments that all yielded similar results. D, LCMV-specific CD8+ T cells support the induction of CTLs against OVA257–264–expressing EG7 lymphoma. Tumor-bearing mice with memory-type LCMVgp33–41–specific CD8+ T-cell responses were injected s.c. with dendritic cells loaded with OVA257–264 peptide, as the EG7 tumor-relevant antigen, either alone or with the LCMVgp33–41 peptide, as a tumor-unrelated heterologous helper epitope. Similar results were obtained in an additional experiment.