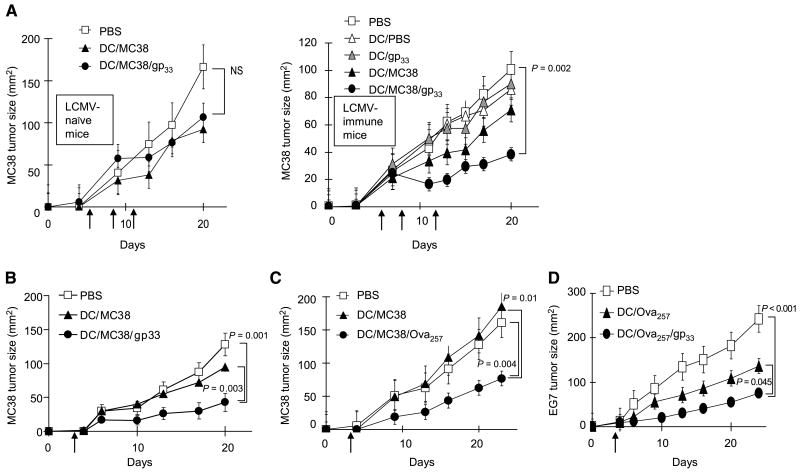
Figure 3.
Heterologous CD8+ T cells support the therapeutic activity of cancer vaccines. A, memory-type LCMVgp33–41–specific CD8+ T cells promote the therapeutic effects of vaccination against day 5 established MC38 tumors. MC38-bearing C57BL/6 mice (n = 5 per group), either naïve (left) or carrying memory-type (week 4) LCMVgp33–41–specific CD8+ T-cell responses (right), were inoculated with MC38 tumors on day 0 and were treated on days 5, 9, and 11 after tumor inoculation. B to D, memory-type CD8+ T cells specific for tumor-unrelated antigens enhance the therapeutic effects of cancer vaccines (single vaccination model). Day 3 tumor-bearing mice (B, n = 10 per group; C and D, n = 5 per group) with memory-type responses against tumor-unrelated heterologous helper antigens were injected s.c. with PBS as a negative control (□), dendritic cells loaded with tumor antigen alone (MC38 tumor lysate or OVA257–264 in the EG7 model, ▲), or the relevant tumor antigen plus a tumor-irrelevant heterologous helper epitope (●). B, memory-type LCMVgp33–41–specific CD8+ T cells support the therapeutic activity of vaccination against MC38 adenocarcinoma. C, memory-type OVA257–264–specific CD8+ T cells support the therapeutic activity of vaccination against MC38 adenocarcinoma. D, memory-type LCMVgp33–41–specific CD8+ T cells support the therapeutic activity of vaccination against OVA257–264–expressing EG7 lymphoma. Points, mean from one of two separate experiments in each model; bars, SE. The differences between the treatment groups were evaluated by ANOVA. NS, nonsignificant differences (P > 0.05).