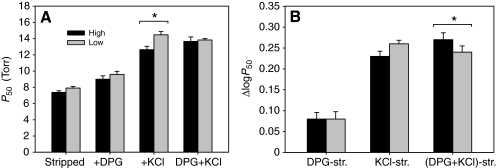
Fig. 5.
O2 binding properties of deer mouse Hbs at pH 7.40 (±0.02) and 37°C in the presence and absence of allosteric cofactors ([Cl–], 0.10 mol l–1; [NaHepes], 0.1 mol l–1; DPG/Hb tetramer ratio, 2.0; [Heme], 0.10–0.16 mmol l–1). (A) P50 (the PO2 at 50% saturation of the heme groups) values (means ± s.e.m.) for stripped hemolysates in the absence of added anions, in the presence of DPG alone, in the presence of Cl– ions alone (added as KCl), and in the combined presence of both anions. (B) Allosteric effect of DPG and Cl– ions on Hb–O2 affinity, expressed as the difference in log-transformed P50 values (means ± s.e.m.) for stripped hemolysates (str.) in the presence and absence of added anions. The ΔlogP50 value measures the extent to which Hb–O2 affinity is reduced in the presence of a given allosteric effector. Asterisks denote statistically significant differences between samples of highland and lowland mice.