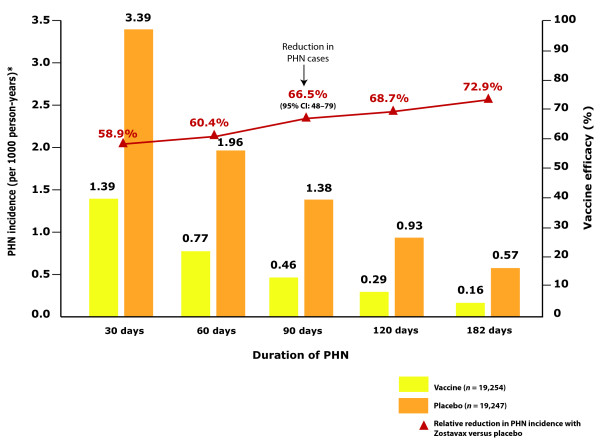
Figure 5.
Duration of pain in vaccine recipients in the Shingles Prevention Study [70]. The graph compares vaccine recipients who developed herpes zoster and post-herpetic neuralgia (PHN; 315/19,254 recipients) with placebo recipients who developed disease (641/19,247 recipients). Compared with placebo, Zostavax reduced PHN incidence defined as pain at different cut-off times for the duration of pain. Pain persisting at 90 days was reduced by 67%. * For the total population and the sub-groups stratified according to sex, the incidence of PHN in each treatment group (vaccine or placebo) was the weighted average of the observed incidence of PHN stratified according to age group, with weights proportional to the total number of person-years of follow-up in each age group.