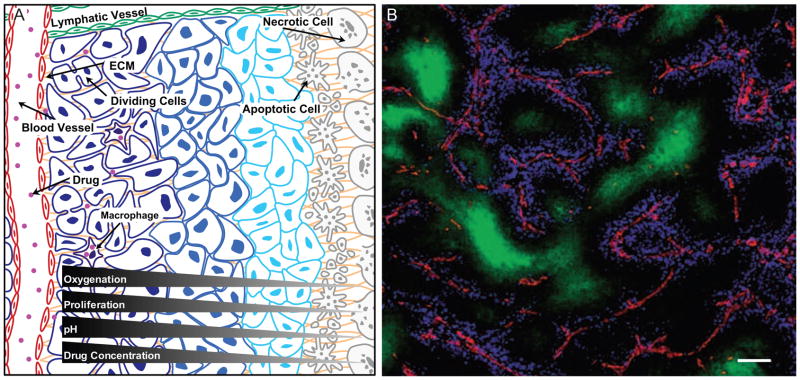
Figure 5. Tumor tissue organization influences drug response.
(A) The overall architecture of the tumor has a direct effect on the ability of a cancer drug to penetrate the tissue and reach the cancer cells. Abnormal leakage from blood vessels together with insufficient lymphatic drainage, especially from the middle of tumors, contribute to increased interstitial pressure in the tumor tissue that inhibits penetration of drugs into the deeper areas of the tumors. Cancer drug penetration is also limited by their binding to ECM proteins, such as collagen, or their uptake by stromal cells, such as macrophages. Cancer cells furthest away from the blood vessel not only are exposed to the lowest drug concentrations, but also receive the lowest amounts of nutrients and oxygen from the circulation and therefore have the lowest proliferative index. As many cancer cell drugs preferentially target actively proliferating cells, this effect contributes to the inability of drugs to target cells in hypoxic areas. Cancer cells further from the blood vessel are also exposed to a low pH microenvironment where many cytotoxic drugs become inactive. Thus, the organization of the tumor tissue results in limited drug availability and efficacy in the hypoxic areas of tumors, areas speculated to contain some of the most aggressive cancer cells. The changing microenvironment in different parts of the tumors therefore results in an apparent difference in drug sensitivity (indicated by the changing color of the cancer cells).
(B) Distribution of doxorubicin in vivo. Section from a mouse mammary tumor showing the distribution of doxorubicin (blue) in relation to tumor blood vessels (red) and regions of hypoxia (green). Note that doxorubicin is found only in close proximity to tumor blood vessels. Scale bar: 100 μm. Reprinted with permission from Primeau et al., 2005.