Figure 6. Translation of polymorphic packing effects to the histamine-binding site.
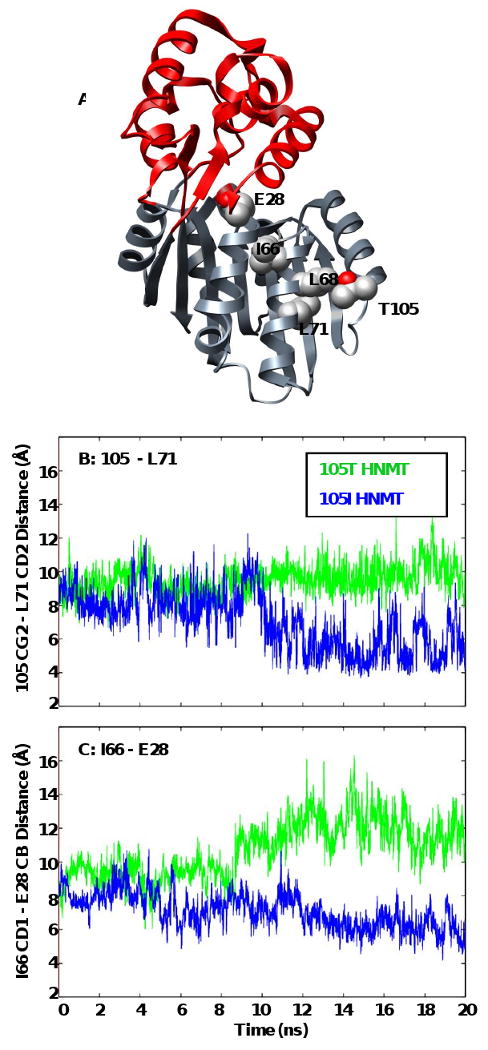
Secondary structures surrounding the polymorphic residue are more susceptible to changes in orientation brought on by altered contacts between L71 and S72 with the larger Ile side-chain. These structural changes are translated to the histamine-binding site via a new contact between α3 (I66) and α2 (E28). (A) Ribbon diagram of HNMT showing the location of residues 105, L71, L72, I66 and E28. Side-chains are shown in space-filling representation and colored by atom. Plots of (B) 105 CG2 – L71 CD2 (α3), and (C) I66 (α3) – E28 (α2, catalytic trio) contact distances for the 105T (green) and 105I (blue) HNMT simulations at 37°C.
