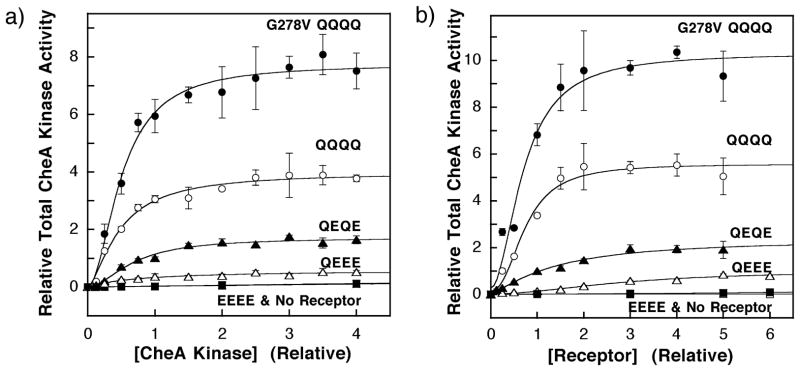
Figure 6.
(A) The effect of increasing concentration of CheA on the kinase activities of reactions containing fixed concentrations of receptor. The in vitro assay of receptor-coupled kinase activity was used to monitor the increase in signaling complex formation upon the titration of CheA into reactions containing 2 μM receptor monomer. The soluble components of the chemotactic signaling complex were covaried at a fixed molar ratio of 1:4:20 for CheA to CheW to CheY, such that CheW and CheY are always present in excess of CheA. Measured kinase activities were normalized relative to that of the wild-type (QEQE) receptor under standard assay conditions (0.5 μM CheA kinase monomer), such that both the relative kinase activity and relative CheA concentration of this reaction were set equal to unity. The resulting data were best-fit by a Hill model that yielded an apparent dissociation constant ( ) and an asymptotic kinase activity in the absence of attractant ( ) as summarized in Table 2. The fit provided Hill coefficients that are difficult to interpret due to the complexities of signaling complex assembly (see the text): H = 1.0 ± 0.3 for QEEE, 1.8 ± 0.4 for QEQE, 1.5 ± 0.2 for QQQQ, and 1.8 ± 0.3 for G278V/QQQQ. The EEEE modification state (filled boxes) showed no significant activity above that of membranes containing no overexpressed receptors (underlying open boxes), preventing measurement of parameters for this state. (B) The effect of increasing concentration of receptor on the kinase activities of reactions containing fixed concentrations of CheA kinase (0.5 μM), CheW (2 μM), and CheY (10 μM). Measured kinase activities were normalized relative to that of the wild-type (QEQE) receptor under standard assay conditions (2.0 μM receptor monomer), such that both the relative kinase activity and relative receptor concentration of this reaction were set equal to unity. The resulting data were best fit by a Hill model that yielded an apparent dissociation constant ( ) and an asymptotic kinase activity in the absence of attractant ( ) as summarized in Table 3. The fit provided Hill coefficients that approach 2.0 but are difficult to interpret due to the complexities of signaling complex assembly (see the text): H = 1.9 ± 0.6 for QEEE, 1.3 ± 0.3 for QEQE, 2.3 ± 0.5 for QQQQ, and 1.9 ± 0.5 for G278V/QQQQ. Other details are as for A.