Abstract
Classical αβ T cells protect the host by monitoring intracellular and extracellular proteins in a two-step process. The first step is protein degradation and combining with a major histocompatibility complex (MHC) molecule leading to surface expression of this amalgam (antigen processing). The second step is the interaction of the T cell receptor (TCR) with the MHC-peptide complex leading to signaling in the T cells (antigen recognition). The context for this interaction is a T cell-antigen presenting cell (APC) junction known as an immunological synapse if symmetric and stable and that we have referred to as a kinapse if it is asymmetric and mobile. The physiological recognition of ligand takes place most efficiently in the filamentous (F)-actin rich lamellipodium and is F-actin dependent in stages of formation, triggering and myosin II dependent for signal amplification. This review discussed how these concepts emerged from early studies on adhesion, signaling and cell biology of T cells.
Introduction
The adaptive immune system is a sensory organ that monitors our inner spaces for evidence of infection or cancer, regulates steady state microbiota and avoids self injury (Krogsgaard and Davis, 2005). The primary filter for this sensor is the dendritic cell (DC), which samples tissue spaces and interfaces for novel macromolecular information (Steinman et al., 2003). DC respond to tissues injury and detect conserved microbial structures leading to changes in DC signals to T lymphocyte (T cells), to shape an appropriate response (Trombetta and Mellman, 2005; West et al., 2004). The largest part of the information is in the form of proteins broken down into peptides that form complexes with surface molecules of the major histocompatibility complex (MHC-peptide complexes) that allow DCs to share this information with T cells expressing the T cell antigen receptors (TCR). An individual has a few hundred T cells that can detect any foreign MHC-peptide complex with single molecule sensitivity (Irvine et al., 2002; Sykulev et al., 1996). This sensitivity evolved by necessity because although the DC may express up to a million MHC molecules, it also samples thousands of proteins, most of which are self-proteins (Trombetta and Mellman, 2005). Thus, each T cell that contacts a DC needs to sort through this huge complexity of ligands and then focus on a few tens or hundreds of ligands that bind the TCR. This ultrasensitive process is still poorly understood, but clues are being discovered at an accelerating rate such that some critical answers are on the horizon. In the 1980’s it was shown that antigen recognition and actin dependent adhesion were integrated processes (Dustin and Springer, 1989); in the 1990’s it was discovered that the actin rich lamellipodium was the most sensitive part of this sensitive cells (Valitutti et al., 1995a); and in the present decade we and others have begun to examine single molecule dynamics of TCR signaling complexes (Douglass and Vale, 2005). This review will describe the cellular context of TCR signaling reactions, which include an important niche based on F-actin rich lamellipodia that can be elaborated in motile and arrested cells.
Adhesion and antigen recognition are integrated by F-actin dependent mechanisms
The TCR and adhesion molecules were identified by antibodies in the same burst of activity to discover the receptors involved in lymphocyte function by screening for inhibitors (Sanchez-Madrid et al., 1982; White et al., 1983). The last piece of the molecular puzzle, the structure of an MHC-peptide complex, was determined in 1987 (Bjorkman et al., 1987). This structure clarified the highly competitive nature of binding short peptides to the MHC molecule in a relatively stable manner (Babbitt et al., 1985). Thus, each TCR is locked into recognizing a small number of antigenic structures and each MHC molecule presents a single peptide. DCs use a limited numbers of any single MHC-peptide ligand to search through a vast repertoire of T cells. Thus, powerful mechanisms are needed to coordinate the search and response. Early work on the T cell signaling response to TCR-MHC-peptide interactions revealed rapid elevation of and protein kinase C activation and cytoplasmic Ca2+ down-stream of a tyrosine kinase cascade (Samelsonet al., 1986). Parallel studies on the adhesion molecule LFA-1 demonstrated that it was regulated by an F-actin and energy dependent mechanism that could be activated by phorbol esters (Marlin and Springer, 1987). LFA-1 was found to mediate adhesion by binding to a family of adhesion molecules including ICAM-1, whose expression reflects the innate immune activation of tissues (Dustin et al., 1986). Resting T cells are non-adhesive to ICAM-1 coated surfaces when freshly isolated from peripheral blood, but become more adhesive to ICAM-1 after triggering of TCR signaling (Dustin and Springer, 1989). The time course of adhesion activation closely followed the time course of TCR signaling: a process of “inside-out signaling” (Dustin and Springer, 1989). In the case of LFA-1, a candidate molecular mechanism for this activation was binding of talin to the cytoplasmic domain of LFA-1 (Smith et al., 2005). Talinis recruited to sites of LFA-1 interaction with ligands on APCs and this is a more sensitive process with respect to antigen dose than cytokine production or proliferation (Kupfer and Singer, 1989). The parallel field studying lymphocyte trafficking revealed that LFA-1 can also be acutely activated by G-protein coupled receptors that are associated with lymphocyte homing (Lawrence and Springer, 1991). Thus, LFA-1 regulation is a cornerstone in both body-wide navigation of lymphocytes through vascular interaction and the coordination of antigen recognition and strong, transient adhesion leading to the immunological synapse in tissues.
Specific signaling pathways are required for inside out signaling from TCR to LFA-1. The adapter molecule ADAP and its partner SKAP-55 have been shown to contribute to about half of TCR triggered adhesion (Peterson et al., 2001). This process is integrated with F-actin at both the level of the TCR signal as discussed below and directly through the interactions of ADAP through Ena-VASP family members, which are involved directly in actin polymerization and contribute to regulation of Arp2/3 (Krause et al., 2000). SKAP-55 forms a complex with RIAM (Menasche et al., 2007), which binds to active Rap-1, a small G-protein related to Ras that is required for regulation of adhesion in lymphocytes. Activation of Rap-1 downstream of tyrosine kinase cascades requires a guanine nucleotide exchange factor (GEF) called C3G. TCR signaling activates C3G by a cascade involving Vav, a GEF for Rac (Krawczyk et al., 2002). Recent evidence suggests that Rac activates the WAVE2 complex, which activates Abl and CrkL-C3G to increase active Rap1 (Nolz et al., 2008). The Rap-1-RIAM complex then contributes to activation of talin, which directly binds to the β2 cytoplasmic domain in the critical hinge region to activate and cluster LFA-1 (Smith et al., 2005; Tadokoro et al., 2003; Wegener et al., 2007). This pathway accounts for the strong correlation of LFA-1 dependent cell-cell adhesion and talin accumulation at the interface.
Another connection to F-actin is observed in the APC. DC mediated antigen presentation is an active process that requires small G-proteins of the Rac family and intact F-actin (Al-Alwan et al., 2003; Benvenuti et al., 2004). This may be in part due to the interaction if ICAM-1 with the actin cytoskeleton, in part via ERM family members.
The immunological synapse
Norcross and Paul first discussed the idea of an immunological synapse with the main “synapse-like” features being a role for Ca2+ elevation, adhesion and directed secretion and polarity (Norcross, 1984). There are a handful of studies in the 1980’s and 90’s that provide quantitative data on cytoskeletal organization, secretory apparatus polarization and Ca2+ signaling in T cell conjugates with B cells e.g. (Geiger et al., 1982; Kupfer et al., 1986; Poenie et al., 1987), in addition to above mentioned studies on adhesion. The classical picture is of cell pairs with a T cell stably appended onto the often-larger target cells like an apse on a building-which is the origin of the term synapse. In one study, a distinct annular adhesive domain and central secretory domains were resolved by electron microscopy (Schmidt et al., 1988), which foreshadows the later work by Kupfer.
The molecular organization of these interfaces was not revealed until advances in imaging in the 1990’s led to the definition of discrete localization of TCR and LFA-1 and a more specific definition of immunological synapse was proposed (Dustin et al., 1998). Springer proposed in 1990 that LFA-1 and TCR would be segregated in adhesive interfaces because they are different sizes and it would be impossible for TCR to reach the MHC in a adhesive interface mediated by LFA-1 and it would be similarly difficult for LFA-1 to squeeze into an interface defined by the smaller CD2-CD58 adhesion system, which would in turn be ideal for the TCR (Springer, 1990). Springer further proposed that exclusion of large phosphatases like CD45 might be important for tyrosine phosphorylation down-stream of the tyrosine kinase cascade. This concept has been refined and tested and seems likely to be an important part of the signaling niche for the TCR (Choudhuri and van der Merwe, 2007; Varma et al., 2006). Springer didn’t speculate on the scale over which this segregation process would take place. The initial answers to this question were surprising. Kupfer examined fixed T cell-B cell conjugates and revealed a large central TCR cluster that was also rich in PKC-θ, defined as the central supramolecular activation cluster (cSMAC) (Monks et al., 1998)(Table 1). A ring of LFA-1-ICAM-1 interactions and talin surrounds this central cluster, defined as the peripheral supramolecular activation cluster (pSMAC). Each of these areas contained thousands of molecules in what was apparently a large non-covalent functional network-a supramolecular assembly. Kupfer later defined the distal supramolecular activation cluster as the outermost structure, enriched in CD45, although this will be discussed further below. The distal pole complex was described by Burkhardt and may be important for sequestering specific negative regulators like SHP-1 (Cullinan et al., 2002). TCR microclusters will be discussed at length. Table I outlines the compartments associated with stable, antigen specific T cell-B cell conjugates.
Table 1.
Components of the Immunological synapse
| SMAC | T cell | APC |
|---|---|---|
| Central (cSMAC)a | TCR (variable), LBPA* | MHC-peptide (variable) |
| Central (cSMAC)b | PKC-θ, CD28 | CD80 |
| Peripheral (pSMAC) | LFA-1, CD2, Talin, F-actin | ICAM-1, CD48/CD58 |
| Distal (dSMAC) | CD45?, CD4?, F-actin | ? |
| Distal Pole Complex | CD43, moesin, SHP-1, PKCζ | N.A. |
| TCR microclusters | TCR, Lck, ZAP-70, SLP-76, LAT, Grb2… | MHC-peptide(s) |
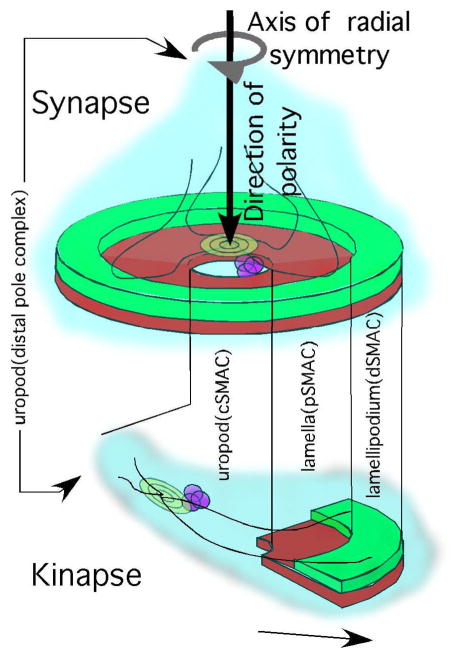
Parallel studies with supported planar bilayers presenting adhesion molecules revealed similar phenomena in which a single adhesion system would establish a cooperative supamolecular cluster and mixed systems would segregate into spatially discrete SMACs. We examined the organization of the CD2-CD58 and LFA-1-ICAM-1 interactions in contacts in Jurkat T cells on planar bilayers containing ICAM-1 and CD58 (Dustin et al., 1998). We proposed that the “specialized junction, cell polarization, and positional stability” of the symmetric contacts were similar to a neural synapse and proposed that stable T-APC interfaces should be defined as “immunological synapses”. A planar bilayer based system using MHC-peptide complexes and LFA-1 fully recapitulated Kupfer’s findings and provided insight into the dynamics of these structures, which were not formed en bloc, but evolved from very distinct early intermediates by an F-actin dependent transport process (Grakoui et al., 1999)(Fig 1). The immunological synapse has an axis of radial symmetry with its center at the cSMAC (Fig 1). At the same time, we can view the synapse as having a direction of polarity along the length of this axis with a proximal and distal pole relative to the APC containing distinct molecules (Fig 1). The function of the immunological synapse pattern is most transparent for cytotoxic T cells where the F-actin free cSMAC serves as a secretory domain and the pSMAC serves as a retaining wall (Beal et al., 2008; Stinchcombe et al., 2006). The importance of directed secretion is supported by studies with human patients with deficiencies in granule transport to the synapse that lead to defects in killing (Baetz et al., 1995). Stabilization of the pSMAC of CD4+ cytotoxic T lymphocyte (CTL) with a PKC-θ inhibitor quantified increased killing, the first evidence of for an advantage of an intact pSMAC (Beal et al., 2008). Structures similar to pSMACs also form between T cells in homotypic aggregates and are important for intensification of cytokine signals during T cell expansion (Sabatos et al., 2008). The symmetric immunological synapse may serve equally important functions in tolerance induction, priming and differentiation as discussed below.
Figure 1.
Synapses vs Kinases. Schematic of multilayer actin cytoskeleton in the periphery of the immunological synapse. Based on (Giannone et al., 2007). Centripetal action flow is involved in antigen gathering. When this system is symmetric the cell forms a stable synapse. When it’s asymmetric the cell gains net traction and crawls using the same machinery. The cSMAC of a synapse forming cells is similar to the uropod of a migrating cell in that the amounts of F-actin are relatively low and integrins are disengaged, but otherwise these structures are very different and this is one of the most striking differences between the synapse and kinapse modes of signal integration.
The importance of lamellipodia in T cell sensitivity-kinapses
Between chemokine dependent extravasation and MHC-peptide dependent immunological synapse formation T cells rapidly migrate in DC networks of T cell zones (Bajenoff et al., 2006). Rapid migration by T cells can be fully reconstituted with solid phase CCL21, a CCR7 ligand, in vitro (Woolf et al., 2007). This involved polarization and formation of a leading edge that takes the form of a flattened lamellipodium on CCL21 and ICAM-1 coated surfaces (Huang and Dustin, unpublished data). LFA-1 is not required for mobility in T cell zones (Woolf et al., 2007), but LFA-1 increases sensitivity to MHC-peptide complexes up to 100-fold (Bachmann et al., 1997) and ICAM-1 is required for antigen specific arrest in vivo (Scholer et al., 2008). The lamellipodium is a sensory structure and has been shown to be the most sensitive part of a T cell by ~40-fold in elegant studies with laser traps and anti-CD3 coated beads (Negulescu et al., 1996; Wei et al., 1999). Valitutti has also described the probing behavior of lymphocytes undergoing sustained signaling on APCs (Valitutti et al., 1995a). Another important experiment is the demonstration that TCR signaling is continually being renewed by new TCR-MHC-peptide interactions such that blocking the access to new MHC-peptide ligands with MHC antibodies immediately block signaling (Valitutti et al., 1995a).
Lamellipodia are coordinated by small G-proteins including Rac-1, which activates the WAVE2 complexes leading to dynamic actin in the periphery of nascent immunological synapses (Nolz et al., 2006). HS-1, a lymphocyte-specific cortactin is important for forming lamellipodia (Gomez et al., 2006). EVL, a hematopoietic cell member of the ENA-VASP family, is found at the tips of projections touching the APC (Lambrechts et al., 2000). Activation of these systems is not antigen specific and similar structures are induced during non-antigen specific engagement of adhesion molecule like LFA-1 by ICAM-1 (Smith et al., 2003) and CD2 by CD58 (or CD48 in the mouse) (Kaizuka et al., 2009). In response to TCR signals, the SLP-76 signaling module recruits and activates Vav, an exchange factor for Rac and Cdc42. Rac then activates WAVE2 and Cdc42 activates WASP. Both WAVE2 and WASP activate the Arp2/3 complex to generate branched actin networks. WAVE2 is associated with lamellipodial actin (Nolz et al., 2006), whereas WASP is associated with ventral projections such as podosomes and invadipodia (Carman et al., 2007). Behind the lamellipodium is the lamella, which has also been referred to as a focal zone in migrating T cells (Smith et al., 2005). These zones are rich in adhesion sites and integrin binding adapter talin, which is also a marker of the pSMAC. T cell may arrest migration and form a stable synapse, or alternatively, they may continue to migrate and integrate signals on the move. This rapid migration during antigen recognition contrasts with the stable synapse and has different functional consequences.
Rapid migration during antigen recognition is observed in T-DC interactions in vitro early stages of T cell interaction with low dose agonist stimuli in vivo (Henrickson et al., 2008) and with low potency TCR stimuli (Skokos et al., 2007). There are at least two explanations for these mobile junctions or “kinapses”(Dustin, 2008). Certain chemokinetic signals, such as CCL21 signals, can compete with antigen stop signals (Bromley et al., 2000). The ability of T cells to migrate for some period followed by arrest may relate to signaling thresholds or time dependent down-regulation of CCR7. This gives the T cells flexibility in looking at different amounts of antigen on DCs-a few DCs with high amounts of MHC-peptide can stop T cells whereas many DCs with few antigen can sustain signaling until the T cells resets its sensitivity and can arrest (Henrickson et al., 2008). Weaker MHC-peptide never overcome the chemokinetic signals and continually migrate in DC networks (Skokos et al., 2007). T cells encountering these weaker ligands cannot arrest in the steady state and only integrate signals through kinapses. This mode of signal integration is sufficient to induce an alternative mode of tolerance induction when the antigen is presented on many DCs through scavenger receptor DEC-205. The failure of some weaker self-antigens to stop T cells may contribute the failure of tolerance induction and the development of autoimmuity (Zehn and Bevan, 2006). In autoimmunity the combination of strong innate signals and weak TCR signals can lead to robust responses, although there are differences in the kinetics of T cell release from lymph nodes and fitness of the cells that may limit memory generation autoimmunity (Zehn and Bevan, 2006). Thus, a migrating T cell sensing MHC-peptide complexes at its leading edge can integrate signals and make decisions about tolerance to abundant antigens, but not rare self-antigens.
Building a dynamic synapse
How do T cells form a synapse? T cells stopped by MHC-peptide complexes remained highly dynamics with prominent cycles of extension and retraction around a pivot point (Dustin et al., 1997; Valitutti et al., 1995a). Studies on planar bilayers reveal what appears to be an early spreading, receptor engagement and contractile process that form the cSMAC over a period of 5 minutes and which is F-actin dependent (Grakoui et al., 1999). These results also led to speculation that myosin II based contraction might also be involved because of the substantial decreases in contact size and the speed of receptor cluster movement (Dustin and Cooper, 2000; Grakoui et al., 1999). Similar results were obtained in live cell-cell systems in which TCR in small clusters moved to the center to form the cSMAC (Krummel et al., 2000). However, these authors eventually rejected the hypothesis that myosin II is required for this contraction and transport process because knockdown of myosin IIA did not appear to eliminate the ability to form a synapse with an APC (Jacobelli et al., 2004). More recent studies in the planar bilayer model actually supported the hypothesis that myosin IIA is important for microcluster transport and signal amplification in that system and for signal amplification in cell-cell models (Ilani et al., 2009). It is possible that there are alternative mechanisms to form a cSMAC in cell-cell systems that involve directed vesicular transport, which would be myosin II independent (Das et al., 2004). Nonetheless, the extension of an APC embracing lamellipodium is observed in cell-cell systems (Antón et al., 2002; Tskvitaria-Fuller et al., 2003). In Jurkat T leukemia cells, spreading on anti-CD3 coated surfaces form a well organized F-actin ring at early time points, and that this ring appear to break up after a contractile phase (Bunnell et al., 2001). These studies suggest that the initial contact expansion is mediated by F-actin driven protrusion, which then shift to a more dynamic extension-retraction process during sustained signaling. In fact the pivoting process observed with solid phase MHC-peptide complexes suggest that the stop was based on loss of persistent polarity. What could account for the ability of cells to rapidly transition from migration to arrest?
Quantitative analysis of TCR dynamics and cell spreading provided insight into how the stable and dynamic characteristics of the synapse are reconciled. Observations of contact areas on planar bilayers with total internal reflection fluorescence microscopy (TIRFM) reveal that the dSMAC is a radial lamellipodium. First, TCR in cells form synapses over a period of 30 minutes and even after cSMAC formation, sub-micron TCR clusters continue to form in the periphery (Varma et al., 2006). These small TCR microclusters forming later during stimulation are invisible to wide-field imaging on bilayers or cell-cell system. In fact, the continual formation of small microclusters to sustain signaling has only recently been observed in cell-cell systems using a combination of laser tweezers and spinning disk confocal microscopy (Oddos et al., 2008). This sustained movement of small TCR clusters suggested that some centripetal F-actin flow continued in the stable synapse.
How can one define a lamellipodium quantitatively? The Sheetz and Wiggins labs developed methods based on loading cells with a fluorescent dye that fills the cytoplasm and then use TIRFM to image the dynamic footprints of the cell. Edge tracking algorithms quantify the advances and retraction of the edges and modify this to compensate for rapid movement of lymphocytes forming kinapses. This data can then be subjected to graphic and correlation analysis to compare the contact area dynamics between cell types (Dobereiner et al., 2006; Sims et al., 2007). Our collaborative studies revealed that the outer edge of the immunological synapse displayed an evolutionarily conserved dynamic pattern of movement referred to as contractile oscillations (Fig 1). This is thought to emerge from cycles of F-actin polymerization and myosin II dependent contraction that periodically pull the membrane protrusion up and back. This is an excellent way to test the mechanical properties of the surface on which cells are interacting. This protrusion and retraction cycle then propagates as a wave around the entire periphery of the cell. These experiments provided strong quantitative evidence the periphery of primary mouse T cells forming synapses or kinapses on planar bilayers with MHC-pepide complexes, ICAM-1 and CD80 form a radial lamellipodium. More recently, Vale and my lab used speckle microscopy to explicitly demonstrate centripetal actin flow in the dSMAC and pSMAC regions (Kaizuka et al., 2007). This F-actin flow is a consequence of the polymerization of F-actin at the protruding edge, which is pushed backwards because the outer edge advances more slowly than actin is added to the growing filament ends. TCR microclusters are transported at about 40% of the speed of the F-actin in the lamellipodium. This suggests that the microclusters bind and dissociate from the actin network. This notion is also supported by studies showing that TCR clusters can navigate around barriers placed in planar bilayers by nanofabrication methods (DeMond et al., 2008). The ability to move around the barriers appears to depend upon periods of transport punctuated by diffusive movement, which allows the microcluster to make progress around diagonal barriers. TCR microcluster transport, contractile oscillations and centripetal F-actin flow support the model that immunological synapses are based on maintaining a radially symmetrical lamellipodium-the same structure Kupfer described as a dSMAC (Freiberg et al., 2002). The dSMAC was reported as having a high concentration of both CD4 and CD45, but TIRFM imaging of these structures on bilayers does not consistently show this. It is likely that the impression of increased CD4 or CD45 in wide-field imaging is based on the lamellipodium having two plasma membrane layers separated by only 100–200 nm of cytoplasmic and thus appearing twice as bright as surrounding single membrane layers in the pSMAC. In fact, by TIRFM CD45 appears relatively uniform in the synapse except for discrete areas of exclusion around TCR microclusters (Varma et al., 2006). Thus, dSMAC is immunological shorthand for a radial lamellipodium.
The pSMAC must also maintain radial symmetry to have a stable synapse because this structure contains most of the integrins that generate traction for movement (Sims et al., 2007). If the symmetry of the pSMAC is broken the cell will migrate, even if a radially symmetrical dSMAC appears to be maintained. The precise mechanism by which symmetry is established or broken is poorly understood. We know that on the supported planar bilayer system WASP is required to maintain a stable synapse and PKC-θ contributes to symmetry breaking (Sims et al., 2007). Symmetry breaking in the contact plane leads to polarity and in a migrating cells this process is based two opposing actin-myosin networks-the lamellipodium dominated by Rac and a high F-actin:myosin II ratio and the uropod dominated by Rho and a low F-actin:myosin II ratio (Xu et al., 2003). Thus, we have speculated that myosin II may be a target in symmetry breaking. PKC-θ phosphorylates WASP interacting protein (WIP) and regulates its interaction with myosin II (Krzewski et al., 2006). WASP also interacts with this complex. One hypothesis is that myosin II activation via WIP phosphorylation leads to contraction of the pSMAC actin network leading to local thinning and breakdown, whereas WASP may either antagonize this myosin II activation or induced ventral actin polymerization to repair the pSMAC and restore symmetry after its been broken. PKC-θ mediated synapse breaking seems like a negative feedback loop because the other major activity of PKC-θ in T cells is activation of NFkB and other transcription factors. Thus, the functional consequences of synapse stabilization as a result of PKC-θ inhibition can only be studied in pre-armed effector cells, which don’t need to activate transcription for function.
Dynamic signaling in microclusters
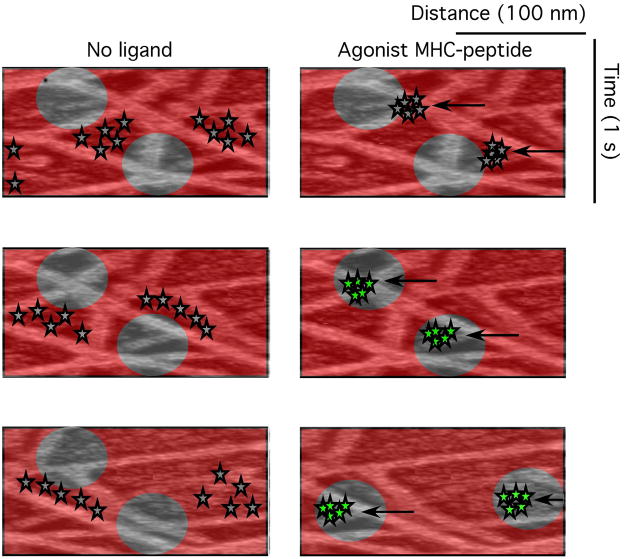
The cSMAC was initially shown to contain PKC-θ and Lck, two kinases that are important for T cell activation (Monks et al., 1998). This suggested that the cSMAC might be involved in sustained signaling. Our early studies on planar bilayers demonstrated that TCR signaling was initiated before the cSMAC was formed based on cytoplasmic Ca2+ elevation at a time point where TCR-MHC-peptide interactions were focused in the periphery (Grakoui et al., 1999). This was more explicitly demonstrated with a specific phopho-Lck antibody, which revealed early TCR signaling in the periphery of the nascent synapse, but not at the cSMAC. This led to the suggestion that the cSMAC might be involved in signal termination (Lee et al., 2003). Computer models suggested that signals in response to weaker ligands might survive in the cSMAC due to a less aggressive attack by ubiquitin ligases. As discussed below this preservation of signaling in the cSMAC may also depend upon a reorganization of F-actin to fill in the normally F-actin depleted central area (Cemerski et al., 2008; Lee et al., 2003). The enigma with peripheral signaling in the immunological synapse was that TCR was not clearly associated with the signaling. This issue was resolved with TIRFM, which greatly increased the contrast of imaging of the cell-planar bilayer interface, as described above, but the discovery of the late submicron TCR microclusters that formed in the dSMAC and moved to the pSMAC over ~ 2 minutes.
The movement of microcluster was interesting for what it said about actin dynamics, but the signaling behavior was even more interesting. Varma repeated the Valittuti experiment of blocking signaling with anti-MHC while observing TCR microclusters and the cSMAC. He found that the TCR microclusters stopped forming and the last microclusters reached the cSMAC about 2 minutes after addition of the antibody, the exact time course with which Ca2+ signaling was eliminated (Varma et al., 2006). Thus, the cSMAC was unable to sustain TCR signaling beyond the 2 minute lifetime of the microclusters, although cSMAC interactions persisted for many minutes. These experiments implicated TCR microclusters in signaling by way of kinetics and complemented experiments that were performed subsequently demonstrating co-localization of TCR microclusters with signaling molecules including ZAP-70, LAT and SLP-76 in live cells and activated ZAP-70, LAT and Lck in fixed cells (Campi et al., 2005; Yokosuka et al., 2005). The movement of these microclusters was a clue to the cellular context of the activation process.
How does the Jurkat T cell line model using solid phase anti-CD3 relate to work in primary cells using bona fide MHC-peptide ligands? Signaling of T cells in response to anti-CD3ε is not blocked by depolymerizatin of F-actin (Valitutti et al., 1995a). Similarly, knock-down of the WAVE2 complex completely eliminates lamellipodial F-actin, but doesn’t inhibit early T cell signaling (Nolz et al., 2006). All but the largest TCR microclusters formed with MHC-peptide ligands are dissolved by depolymerization of F-actin (Varma et al., 2006). Nonetheless, the spreading of Jurkat cells on surfaces coated by anti-CD3ε is accompanied by the formation of a dramatic lamellipodial F-actin ring (Bunnell et al., 2001) and the formation of many discrete TCR microclusters throughout the interface that require F-actin to form in the normal manner (Bunnell et al., 2002). These structures recruit ZAP-70 and SLP-76 and, interestingly, the SLP-76 foci dissociate from the ZAP-70 rich TCR clusters and stream toward the center of the interface based on a mixture of F-actin and microtubule dependent transport processes and appear to engage in sustained signaling even after internalization (Barr et al., 2006; Bunnell et al., 2006) Nguyen et al, 2008). This behavior is strikingly similar to the observed movement of MHC-peptide based TCR microclusters, which occurs in the plane of the plasma membrane in the synapse (Varma et al., 2006). However, the anti-CD3ε driven TCR clusters become systematically F-actin independent after formation (Douglass and Vale, 2005). The inclusion of β1 integrin ligands like fibronectin on the surface with anti-CD3ε results in stabilization of the SLP-76 interaction with the TCR clusters and also appears to dampen F-actin dynamics (Nguyen et al., 2008). Integrin ligands generally enhance TCR signaling (Shimizu et al., 1990) and this study suggests that one component of this effect may be stabilization of TCR signaling complexes. There are clearly differences between the polyvalent anti-CD3ε interaction with the TCR complex, which contains two copies of the CD3ε chain (Call et al., 2002), and the monovalent interaction of MHC-peptide ligands with TCR that change requirements for signaling. The transport process in the planar bilayers also creates a very useful lateral segregation of TCR based structures by age with the earliest TCR microclusters in the periphery and the oldest TCR clusters in the cSMAC. Recently, monovalent biotinylation of anti-CD3εhas created a means to achieve such tempero-spatial segregation of TCR signaling complexes by attaching anti-CD3 to planar bilayers with lipid anchored ICAM-1 to form classical synapses with Jurkat cells or polyclonal T cell populations (Kaizuka et al., 2007). This method has contributed to our understanding of the synapse dynamics. We have two quite different ways to study microclusters that are appropriate for different questions and have different degrees of difficulty. It is impossible to say that one is better than the other at this point-it depends upon the experiment. Further study of microclusters in T cell-DC interfaces using advanced confocal methodologies and even in vivo will be needed to drill deeper into T cell signaling (Oddos et al., 2008).
The relationship between TCR microclusters and F-actin suggests that the TCR clusters are formed actively rather than by diffusion trapping by ligand alone. The formation of TCR microclusters is F-actin dependent whether the ligand is a physiological MHC-peptide complex or a CD3 antibody (Bunnell et al., 2001; Campi et al., 2005; Douglass and Vale, 2005; Varma et al., 2006). This also extends to signaling because depolymerization of actin in an established synapse results in rapid cessation of signaling, but does not disrupt larger TCR microclusters or the cSMAC (Varma et al., 2006). The synapse contains a zone of F-actin depletion at the center, corresponding to the cSMAC regardless of whether this structure is filled with TCR (Kaizuka et al., 2007). When TCR clusters reach the actin free zone in the center the signaling process appears to be terminated. The mechanism of signal termination is not known, but may be as simple as the inability to amplify signals in the absence of F-actin. It is not known how the TCR microclusters continue to move to the center of the synapse when the F-actin conveyor belt stops 1–2 μm from the center. This could be a diffusive process or might involve some alternative transport system that remains to be described. For example, the zone of F-actin depletion is observed in cytotoxic T cell synapses and allows close approach of the centrosome to the plasma membrane for efficient directed secretion (Stinchcombe et al., 2006).
It is not clear why F-actin is so important for TCR signal transduction because anti-CD3 stimulation to Ca2+ mobilization and cytokine production doesn’t require intact F-actin (Valitutti et al., 1995a). Similarly, B cells require F-actin to recognize ligands on a surface, but not aggregating ligands in solution. Stimulation by surface presented ligands requires F-actin, Rap1 and CD19 (Depoil et al., 2008; Lin et al., 2008). The model for stimulation of activation of BCR by monovalent ligands in supported planar bilayers is based on diffusion trapping of receptors and ligands in microdomains with multiple BCR and ligands. This mode of clustering is likely driven by F-actin dependent membrane fluctuations and adhesion, perhaps accounting for the role of F-actin in enhancing B cell recognition of solid phase ligands (Carrasco et al., 2004; Tolar et al., 2008). For T cells, the F-actin requirement seems to involve both forming microclusters and then formation of signaling complexes at the TCR clusters. This is based on the observation that many TCR microclusters are stable after F-actin depolymerization, but signaling is still terminated even though the receptor clusters persist. All antigen receptors engage in signaling that activates actin polymerization via the Rac and Cdc42 small G-proteins leading to activation of the WAVE2 complexes and WASp (Barda-Saad et al., 2005; Nolz et al., 2006). In the absence of WAVE2 the lamellipodium at the periphery of T cell contacts with anti-CD3 coated surfaces is lost (Nolz et al., 2006). This does not alter that ability of these cells to initiate early TCR signaling in response to solid phase anti-CD3, but more work is needed to determine if this alters sensitivity to MHC-peptide ligands as expected.
The recent focus on microclusters raises questions about cSMAC-associated signals. Is the cSMAC just for signal termination or is it a site of TCR or other relevant signaling? Is the cSMAC even one compartment? Kupfer defined the cSMAC based on accumulation of TCR and PKC-θ. Dynamic studies using fluorescence recovery after photobleaching (FRAP) revealed that TCR-MHC-peptide interactions are particularly stable in the cSMAC (Grakoui et al., 1999) and that the TCR microclusters that converge to form the cSMAC appear to fuse together into one stable structure on planar bilayers (Varma et al., 2006). This structure is partly shed by the T cell if it breaks the synapse and migrates or wanes over 10’s of minutes in stable synapses. Is this highly stabilized structure in which TCR seem to be shed or degraded all there is to the cSMAC? Analysis of CD28-CD80 interactions in the synapse suggests that there is a second, more dynamic component to the cSMAC that is typically not well resolved, but represents a very distinct sub-cellular compartment (Tseng et al., 2008; Yokosuka et al., 2008). CD28-CD80 interactions colocalize with TCR-MHC-peptide interactions in microclusters. When these mixed microclusters reach the cSMAC, mostof the TCR are incorporated into a stable central cluster, but all of the CD28 remains in an intermediate compartments which is very dynamic and appears to be critical for sustained PKC-θ signaling (Yokosuka et al., 2008). This annular structure sits at the inside edge of the F-actin rich lamella and thus the small amounts of TCR in this compartment may continue to signal to maintain the dynamic CD28-CD80 interactions. Similar segregation of TCR and CD28 is observed in T cell-DC interfaces, although it was not possible to observe the precursor microclusters (Tseng et al., 2008). Blocking TCR signaling with anti-MHC-peptide antibodies rapidly eliminated the bright CD28-CD80 foci, consistent with a continual role for TCR signaling in maintaining this component of the cSMAC. These studies demonstrate that the cSMAC consists of two compartments: one a stable structure enriched in non-signaling TCR destined for shedding or degradation (a) and one highly dynamic structure rich in F-actin, CD28 and PKC-θ (b) (table 1). These compartments are difficult to resolve in cSMACs formedin cell-cell interfaces. The mechanisms by which CD28 and TCR are segregated into these two cSMAC compartments is not known.
A model for TCR triggering
The mechanism of TCR triggering has been an enduring problem. The prevailing model has been based on TCR dimerization or clustering as a triggering modality (Germain, 1997; Weiss and Littman, 1994). This is largely based on models from receptor tyrosine kinases and observations that antibody crosslinking triggers signaling. However, the is an adapter that recruits non-receptor tyrosine kinases to 10 docking sites, the immunotyrosine based activation motifs (ITAMs), in response to ligand binding. With all these docking sites, it is not clear that dimerizing this receptor would increase its activity. Recent studies have provided indirect evidence that monovalent ligands can trigger TCR microclusters and signaling events without directly cross-linking the receptor to form dimers e.g.(Varma et al., 2006). It is interesting at this point to think about how the TCR would sense such a monovalent engagement event? Recent data from the BCR and TCR systems provide insights into accessibility changes in cytoplasmic domains of the receptor during triggering and provide raw material for a draft model that incorporates an early role for F-actin.
The BCR has two signal transduction subunits with one ITAM in each. BCR with a FRET donor at the C-terminus of transmembrane immunoglobulin (sIg) and a FRET acceptor on the C-terminus of Igα have a high FRET signal in the basal, non-signaling state. Remarkably, triggering results in a rapid decrease in the FRET signal, suggesting an “opening” of the complex (Tolar et al., 2005). It is not known what conformational change causes this decreased FRET. One interpretation is based on the model that the ITAMs may interact with the membrane prior to triggering, making the tyrosine inaccessible to kinases and preventing signaling (Aivazian and Stern, 2000). The interaction with the membrane would confine the FRET acceptor associated with the C-terminus of the ITAM bearing subunit to the membrane in proximity to the FRET donor that is kept close to the membrane by the short sIg cytoplasmic domain. Triggering would then involve dissociation of the ITAM from the membrane. A recent study using live cell FRET imaging and structure determination supports the idea that ITAM displacement from the membrane is a critical step in triggering (Xu et al., 2008). This study found that each ITAM in the TCR has a positively charged motif on its N-terminal side that interacts with acidic phospholipids in the inner leaflet and facilitates the docking of both tyrosines in the lipid bilayer where they cannot be phosphorylated. The results suggest that a change in the lipid environment to a more neutral composition is needed for ITAM access by Src family kinases (Douglass and Vale, 2005). How could a single MHC-peptide complex change the lipid environment of the TCR?
Our understanding of the basal distribution of the TCR is constrained by two seemingly contradictory results: evidence for preclustering from electron microscopy (Schamel et al., 2005) and non-correlated monomeric diffusion using fluorescence correlation spectroscopy (James et al., 2007). Long term tracking of Fce receptor movements in the context of actin bundles on rat basophilic leukemia cells shows that receptors can be corralled by actin and diffuse independently at the same time (Andrews et al., 2008). These results suggest that the clusters seen in EM maybe a result of transient confinement on monomeric receptors. Basal fluctuations in TCR density were observed as highly dynamic structures similar in size to microclusters, but which could not be tracked from one frame to the next (Varma et al., 2006). In order to maintain low basal activity the membrane domains in which the TCR moves prior to ligand binding will likely be highly acidic and fluid.
The lamellipodium is a relatively flat membrane region with a dense mesh of branched actin filaments translocating away from the leading edge (Svitkina et al., 1997). The mesh size of the actin gel if relatively fine, with interstitial areas on the order of 100 nm based on electron microscopy analysis of model lamellipodia (Svitkina et al., 1997). This F-actin mesh is similar in scale to the lipids domains that have been identified in membranes as lipid rafts (Sharma et al., 2004). The spatial distribution of acidic lipids like PS on the inner leaflet is not known, but structural constrains suggest that liquid ordered domains should be enriched in neutral phosphatidylethanolamine (Brown and London, 2000). I would speculate that TCR maybe confined to approximately 10% of the plasma membrane composed of disordered, fluid phase and acidic lipids, which are corralled by the mobile actin elements. In the basal state the TCR cannot be individually transported into less acidic liquid ordered domains (Fig 2). Clustering of the receptors by serial ligand binding can stabilize the interaction with the F-actin meshwork and create a sufficient force to overcome resistance to entry into less acidic domains, in which the ITAMs are exposed in the presence of Src family kinases and with depletion of CD45 together lead to triggering of the signaling cascade (Fig 2). It is likely the the “lipid rafts” also anchored to disctinct cortical actin networks and this actin may anchor these domains against movement by the dendritic actin network inivolved in the retrograde flow (Chichili and Rodgers, 2007). This model invokes shearing action of the lamellipodial actin to generate force to overcome energetic barriers, similar to recent models for the role of lateral mechanical forces in integrin activation (Zhu et al., 2008).
Figure 2.
Model for TCR triggering involving translocation between membrane domains with different levels of acidic lipids. The bulk plasma membrane is red (negatively charged) and this would maintain ITAMs buried in membrane (grey). The overlaying actin network of the T cells is represented by an electron micrograph of lamellipodial actin (Svitkina et al., 1997). In the absence of specific TCR ligands, the TCR (grey stars) are loosely corralled by actin network, but diffuse independently within corrals and cannot be forced to enter more neutral rafts because this is unfavorable and the actin can’t exert enough force on one TCR to prevent the TCR from jumping out through hop diffusion (Kusumi et al., 2005). Monovalent MHC-peptide agonists induce direct clustering of TCR and stronger linkage to actin, which then produces enough force to move the TCR microcluster into a neutral domain where CD45 is excluded and active Src can diffuse in to phosphorylated the dissociated ITAM (green stars). This more neutral lipid domain, which itself can be anchored to the cortical cytoskeleton can be intermittently dragged with the TCR microcluster to sustain signaling until the system reaches the cSMAC.
The specific adapters that link TCR to F-actin are not well described. The complex may include NCK, SLP-76, ADAP, SKAP55, EVL, Vav, Rac, Cdc42 and WASP certainly may play a role once signaling is initiated (Barda-Saad et al., 2005; Bubeck Wardenburg et al., 1998; Krause et al., 2000). It has also been suggested that ZAP-70 may play a role through binding of ezrin (Ilani et al., 2007). It appears that TCR signaling in microclusters ceases when the microcluster reaches the actin depleted cSMAC. Since activated TCR appear equipped to generate their own dynamic actin focus, the suppression of this mode of actin dymanics in the cSMAC is likely to be active, rather than a passive dissipation of lamellar actin. Less is known about the basal interactions of TCR with actin that might be available to stabilize a cluster early in the triggering process. This is likely a complex situation. An example of the potential complexity can be seen in interaction networks recently compiled as the integrin adhesome (Zaidel-Bar et al., 2007).
Once the ITAMs are exposed Src family kinases can then initiate phosphorylation by diffusion into the same domain (Douglass and Vale, 2005)(Fig 2). Exclusion of CD45 in the nascent foci would increase the half-life of the phosphorylated ITAM allowing sufficient time for ZAP-70 recruitment (Varma et al., 2006). This cluster may interact more strongly with LAT so that ZAP-70 could phosphorylate LAT and Itk to initiate PLC-g recruitment and initiation of Ca2+ signaling, PKC activation and Ras-GRP recruitment. Integrin microclusters will be interspersed with the TCR microclusters. Integrin dependent signaling will generate additional diacylglycerol for RasGRP by activating phospholipase D and phosphatidic acid phosphatase (Mor et al., 2007). Electron micrographs of membrane sheets suggest that immunoreceptor rich and LAT rich islands only partially mix (Lillemeier et al., 2006; Wilson et al., 2001). The force needed to induce island mixing may be generated by periodic myosin II based contraction. This may account for the role of myosin IIA in the signal amplification from Lck, which is normally activated after myosin IIA knock-down, to ZAP-70 and LAT, which are only weakly activated in myosin IIA knock-down T cells (Ilani et al., 2009).
Where is this going?
The TCR triggering process in microclusters and how this is sustained in a synapse has relevance to T cell decision-making. T cell differentiation is controlled in large part by cytokines and small molecules from APC. There are two independent reports that propose distinct mechanisms for the synapse to influence differentiation. One is the concentration of interferon-γ receptors in the synapse leading to increased T helper 1 (Th1) cell development (Maldonado et al., 2004). IL-4 counteracts this through Stat6 (Maldonado et al., 2004). The other is the control of polarity networks leading to asymmetric cell division to control memory-effector decisions (Chang et al., 2007; Ludford-Menting et al., 2005). A particular challenge is in understanding how the microcluster-based mechanism sustains signaling from small numbers of ligand to drive differentiation. If each microcluster can sustain signaling for 2 minutes as the microclusters translocates from the periphery to the cSMAC. How is signaling sustained with only 10 MHC-peptide complexes? An interesting observation is that although the actin depleted zone at the center of the synapse is always present, the size of the cSMAC is linearly dependent upon the MHC-peptide density such that no TCR cSMAC if formed at the lower limit of MHC-peptide density. Under these conditions our model would posit that MHC-peptide ligand are recycled expensively by long term serial triggering at the F-actin rich cSMAC-pSMAC boundary. This fits with notions of serial triggering initially propose by Valittuti and Lanzavechia (Valitutti et al., 1995b), but would induce very little if any TCR down-regulation because all receptors are needed for sensitive recognition of rare MHC-peptide ligands. The links between TCR signaling and polarity networks include Cdc42 and PKC-ζ. Activation of Cdc42 has been extensively studied in T cells due to interest in immunodeficiencies such as Wiscott-Alrdrich Syndrome. PKC-ζ can be activated in the Par3-Par6 complex by activation of Cdc42, but it is not clear what it means that PKC-ζ is concentrated in the distal pole complex, the part of the T cell farthest from the synapse (Chang et al., 2007). The polarity networks control the positioning of actin, microtubules and intermediate filament and spindles. The dynamic F-actin networks and myosin II seem important at early stages of signaling complex assembly. The actin-myosin system is critical for multiple stages in TCR triggering and sustained signaling. It will be exciting in the future to determine in more detail how the context of TCR signaling allows this process to shape differentiation in parallel with powerful innate signals.
Acknowledgments
I thank Kaushik Choudhuri and David Fooksman for valuable discussions. Due to length constraints not all relevant literature could be cited and I apologize in advance regarding omissions. This work was supported by NIH grants AI43542and the PN2 EY016586, a Nanomedicine Development Center.
References
- Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- Al-Alwan MM, Liwski RS, Haeryfar SM, Baldridge WH, Hoskin DW, Rowden G, West KA. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–4483. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- Andrews NL, Lidke KA, Pfeiffer JR, Burns AR, Wilson BS, Oliver JM, Lidke DS. Actin restricts FcepsilonRI diffusion and facilitates antigen-induced receptor immobilization. Nat Cell Biol. 2008;10:955–963. doi: 10.1038/ncb1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón IM, de la Fuente MA, Sims TN, Freeman S, Ramesh N, Hartwig JH, Dustin ML, Geha RS. WIP Deficiency Reveals a Differential Role for WIP and the Actin Cytoskeleton in T and B Cell Activation. Immunity. 2002;16:193–204. doi: 10.1016/s1074-7613(02)00268-6. [DOI] [PubMed] [Google Scholar]
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122–6131. [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- Barr VA, Balagopalan L, Barda-Saad M, Polishchuk R, Boukari H, Bunnell SC, Bernot KM, Toda Y, Nossal R, Samelson LE. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic. 2006;7:1143–1162. doi: 10.1111/j.1600-0854.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein Kinase C {theta} Regulates Stability of the Peripheral Adhesion Ring Junction and Contributes to the Sensitivity of Target Cell Lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987;329:512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting Edge: Hierarchy of Chemokine Receptor and TCR Signals Regulating T Cell Migration and Proliferation. J Immunol. 2000;165:15–19. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid-and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, Chan AC. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario MC, Barr VA, Koretzky GA, Samelson LE. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 2006;26:7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–599. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chichili GR, Rodgers W. Clustering of membrane raft proteins by the actin cytoskeleton. J Biol Chem. 2007;282:36682–36691. doi: 10.1074/jbc.M702959200. [DOI] [PubMed] [Google Scholar]
- Choudhuri K, van der Merwe PA. Molecular mechanisms involved in T cell receptor triggering. Semin Immunol. 2007;19:255–261. doi: 10.1016/j.smim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- Dobereiner HG, Dubin-Thaler BJ, Hofman JM, Xenias HS, Sims TN, Giannone G, Dustin ML, Wiggins CH, Sheetz MP. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys Rev Lett. 2006;97:038102. doi: 10.1103/PhysRevLett.97.038102. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-Molecule Microscopy Reveals Plasma Membrane Microdomains Created by Protein-Protein Networks that Exclude or Trap Signaling Molecules in T Cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon, tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN. T-cell signaling: the importance of receptor clustering. Curr Biol. 1997;7:R640–644. doi: 10.1016/s0960-9822(06)00323-x. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, Sheetz MP. Lamellipodial actin mechanically links Myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, et al. HS1 Functions as an Essential Actin-Regulatory Adaptor Protein at the Immune Synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. TCR signaling and immunological syanpse stability requires myosin IIA. Nat Immunol. 2009 doi: 10.1038/ni.1723. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- James JR, White SS, Clarke RW, Johansen AM, Dunne PD, Sleep DL, Fitzgerald WJ, Davis SJ, Klenerman D. Single-molecule level analysis of the subunit composition of the T cell receptor on live T cells. Proc Natl Acad Sci U S A. 2007;104:17662–17667. doi: 10.1073/pnas.0700411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The co-receptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J Cell Biol. 2009 doi: 10.1083/jcb.200809136. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci U S A. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Sechi AS, Konradt M, Monner D, Gertler FB, Wehland J. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/Vasodilator-stimulated prosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3ζ during T cell recognition. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- Krzewski K, Chen X, Orange JS, Strominger JL. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–132. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Swain SL, Janeway CA, Jr, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci U S A. 1986;83:6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci U S A. 2006;103:18992–18997. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KB, Freeman SA, Zabetian S, Brugger H, Weber M, Lei V, Dang-Lawson M, Tse KW, Santamaria R, Batista FD, Gold MR. The rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity. 2008;28:75–87. doi: 10.1016/j.immuni.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, Irvine DJ, Schreiber R, Glimcher LH. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 2004;431:527–532. doi: 10.1038/nature02916. [DOI] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol. 2007;27:4070–4081. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, Billadeau DD. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl-and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol (Paris) 1984;135D:113–134. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddos S, Dunsby C, Purbhoo MA, Chauveau A, Owen DM, Neil MA, Davis DM, French PM. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008 doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- Poenie M, Tsien RY, Schmitt-Verhulst A. Sequential activation and lethal hit measured by [Ca++]i in individual cytolytic T cells and targets. EMBO J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ, Krummel MF. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson LE, Patel MD, Weissman AM, Harford JB, Klausner RD. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986;46:1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, Springer TA. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis:LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcon B. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RE, Caulfield JP, Michon J, Hein A, Kamada MM, MacDermott RP, Stevens RL, Ritz J. T11/CD2 activation of cloned human natural killer cells results in increased conjugate formation and exocytosis of cytolytic granules. J Immunol. 1988;140:991–1002. [PubMed] [Google Scholar]
- Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Sharma P, Varma R, Sarasij RC, Ira Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, et al. Opposing Effects of PKCtheta and WASp on Symmetry Breaking and Relocation of the Immunological Synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, De Pereda JM, Ginsberg MH, Calderwood DA. Talin Binding to Integrin {beta} Tails: A Final Common Step in Integrin Activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol. 2005;6:1168–1176. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
- Tolar P, Sohn HW, Pierce SK. Viewing the antigen-induced initiation of B-cell activation in living cells. Immunol Rev. 2008;221:64–76. doi: 10.1111/j.1600-065X.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase Ctheta. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tskvitaria-Fuller I, Rozelle AL, Yin HL, Wulfing C. Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization. J Immunol. 2003;171:2287–2295. doi: 10.4049/jimmunol.171.5.2287. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995a;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995b;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Wei X, Tromberg BJ, Cahalan MD. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proc Natl Acad Sci U S A. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- White J, Haskins KM, Marrack P, Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J Immunol. 1983;130:1033–1037. [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Surviladze Z, Gaudet EA, Oliver JM. High resolution mapping of mast cell membranes reveals primary and secondary domains of Fc(epsilon)RI and LAT. J Cell Biol. 2001;154:645–658. doi: 10.1083/jcb.200104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3 epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang F, VanKeymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]