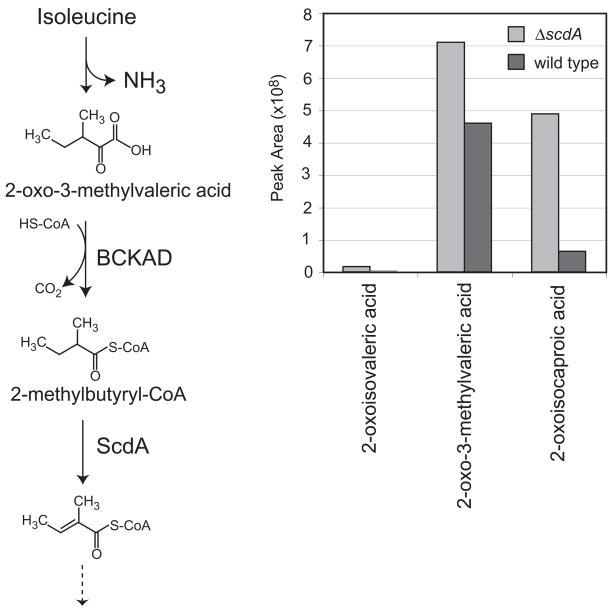
Figure 6.
Accumulation of intermediates of branched-chain amino acid catabolism in the ΔscdA mutant incubated with Ile. A. Detection of intermediates of Val, Ile, and Leu catabolism in culture supernatants of wild type and mutant (ΔscdA) strains of A. nidulans: 2-oxoisovaleric acid, 2-oxo-3-methylvaleric acid, and 2-oxoisocaproic acid. 2-oxocaproic acid was added to culture supernatants as an internal standard prior to sample lyophilization and derivatization. Shown are normalized GC/MS peak area measurements for wild type (A26, dark bars) and ΔscdA mutant (TLMH17, light bars). B. Shown is the proposed Ile catabolic pathway in A. nidulans, based on studies in other eukaryotes (Robinson et al., 1956) and genetic and genomic data for A. nidulans (Maggio-Hall and Keller, 2004). Ile is converted to 2-oxo-3-methylvaleric acid by the action of an unidentified transaminase. Branched-chain α-keto acid dehydrogenase (BCKAD) then generates 2-methylbutyryl-CoA, a substrate for β-oxidation enzymes, including ScdA (as indicated). One complete oxidative cycle yields acetyl-CoA and propionyl-CoA.