Figure 1. Cross-presentation of OVA and gp100 to transgenic T cells was greatly affected by the inhibition or induction of autophagy in the antigen donor cells.
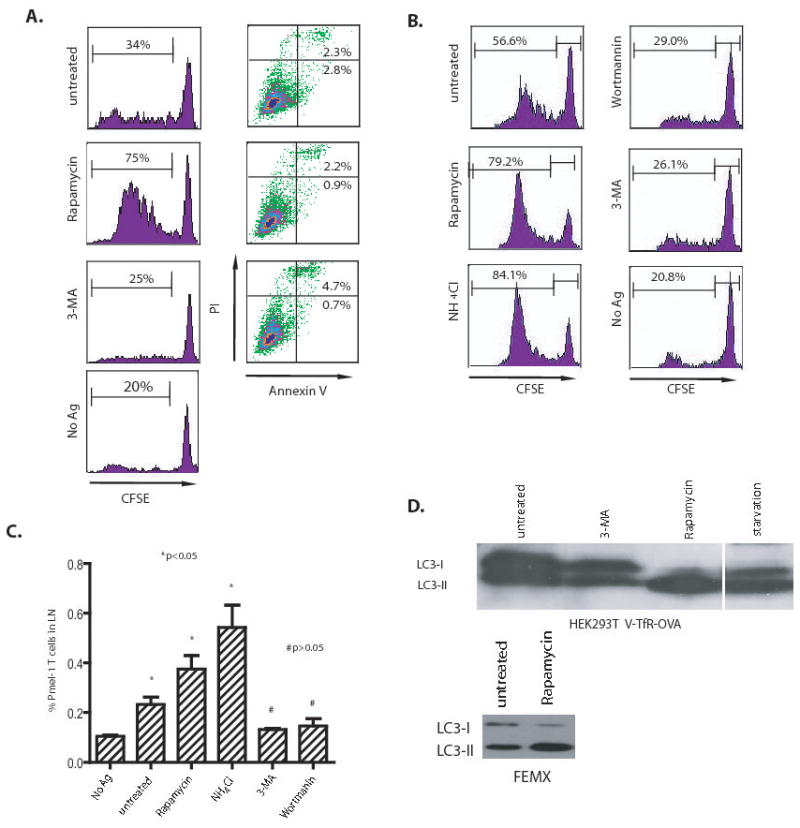
(A) Modulation of cross-presentation of the OVA model antigen in vitro. HEK 293T cells expressing the GFP-OVA fusion protein were treated with the autophagy-inhibitors, 3-MA or wortmanin, or the autophagy-inducer, rapamycin for 18 hours, or subjected to starvation by culturing donor cells in HBSS for 18 hours. PI and FITC-labelled annexin V were used to stain treated cells to measure the levels of apoptosis or necrosis, respectively. To assess cross-presentation, treated tumor cells were irradiated and incubated with DC for 6 hours. CFSE-labeled naive T cells from OT-I TCR transgenic mice were added to the mixture of DC and 293T donor cells and cultured for 4 to 5 days. The dilution of CFSE label in OT-I T cells was determined by flow cytometry. The results are expressed as the percentage of OT-I T cells that had undergone at least one division 4 days after coculture with DC and donor cells. The data represent typical results of three to five independent experiments.
(B) and (C) Modulation of cross-presentation of the melanoma gp100 antigen in vivo. Cells from the human melanoma cell line FEMX were treated with the indicated agents as described above and then injected into the flanks of naïve C57BL/6 mice. CFSE-labelled naïve spleen cells from pmel-1 transgenic mice were adoptively transferred into tumor-bearing mice and lymph nodes draining the tumors were collected at day 6. Both the CFSE profile of pmel-1 CD8 T cells (B) and the percentage of pmel-1 T cells among the lymph node lymphocytes (C) were determined by flow cytometry. Mice that received no tumor or untreated FEMX cells were included as the controls. Each group consisted of 4 mice and the experiment was repeated once with similar results. The difference between treated and untreated groups was significant (*p <0.05); however, the difference between no Ag and 3-MA or wortmannin-treated group was not significant (#p >0.05).
(D) HEK 293T cells expressing OVA antigen or FEMX melanoma cells were treated with 20 nM rapamycin or 10 mM 3-MA for 18 hours. Lysates were prepared from both untreated and treated cells. Lysate from starved HEK 293T cells was included as positive control. Ten microgram of total proteins were loaded and subjected to SDS PAGE and western blot analysis with rabbit anti-LC3 antibody.
